Eosinophilic gastroenteritis (EGE) is a rare disease that mainly affects men in the third or fourth decade of life.1 In 1990, Talley et al.2 defined three diagnostic criteria that still apply today: 1) gastrointestinal symptoms, such as abdominal pain, nausea, vomiting, diarrhea, and bloating,2 2) eosinophil infiltration of any layer or zone of the digestive tract, demonstrated by biopsy,3 and 3) the complete ruling out of other causes of systemic eosinophilia.1 Studies conducted in the United States have found a prevalence varying from 8.4 to 28 per 100,000 inhabitants, with a slightly increasing incidence over the past 50 years.3 A higher socioeconomic level, White race, and excess weight can be risk factors for EGE and familial case reports suggest a possible hereditary component.3
There are three types of disease presentation. Mucosal involvement corresponds to 70% of cases and generally manifests as protein-losing enteropathy. The muscle type accounts for 20% of cases and produces thickening of the gastrointestinal wall with a potential obstructive risk. The rarest form described is the subserosa type (10%), which causes eosinophilic ascites.2 The pathophysiology is not clear. Seventy percent of patients present with eosinophilia and 50% have allergies or are associated with elevated immunoglobulin (Ig) E; said situation suggests a probable immune deregulation in response to an allergic reaction, albeit a triggering allergen is not always identified.3 Even though peripheral eosinophilia is present in the majority of patients, 30% may not present with it, making the diagnosis even more difficult.1
Corticoids are the therapeutic cornerstone, with 20 or 40 mg of oral prednisone taken daily for six to eight weeks. Other medications, such as budesonide 9 mg/day and montelukast 10 mg/day, have also been efficacious for remission induction and maintenance in the majority of reported cases.3 In addition, a controlled elimination diet of six foods with a high allergenic potential can be recommended: milk proteins, soy, wheat, eggs, dried fruits, and fish for at least four to six weeks, with a progressive reintroduction based on tolerance.4,5 In general, treatment progresses favorably, but cases of surgical complications due to obstruction and recurrences that require maintenance treatment with low doses of prednisone (5 to 10 mg daily) to prevent relapse have been described.1,4,5
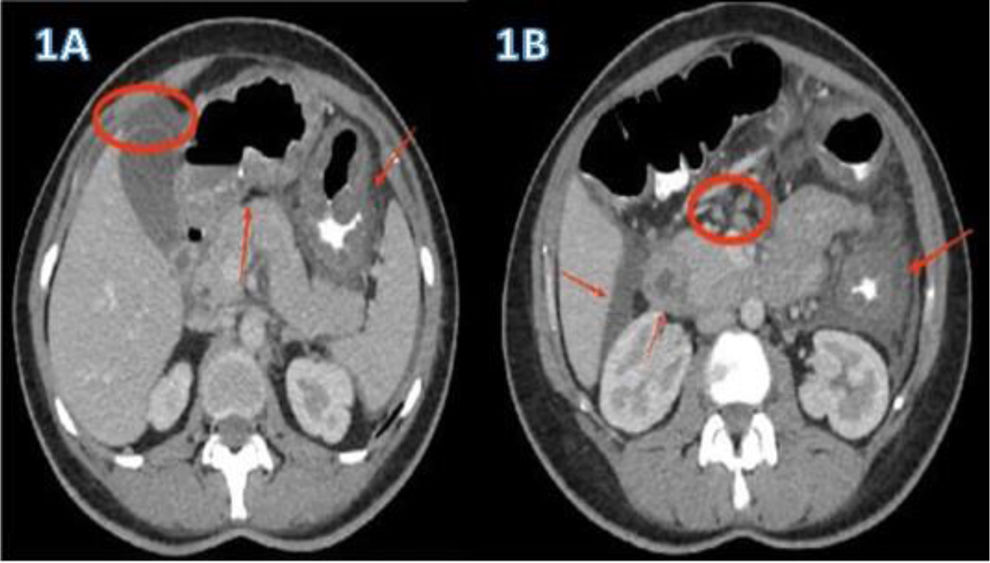
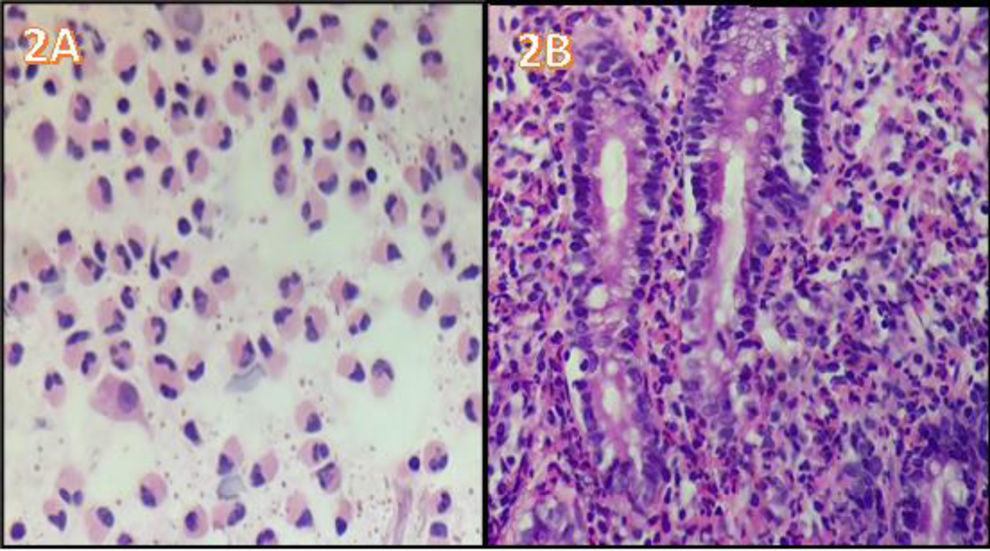
A 40-year-old woman, with a history of allergic rhinitis, was in the late puerperal period. She had not traveled recently or used herbal medicine or a new drug. She arrived at the emergency service because of abdominal pain and increasing abdominal distension, associated with diarrhea and vomiting. Hemogram identified marked eosinophilia (9.460x mm3) with no alteration in any other cell lines. Abdominal ultrasound revealed moderate ascites and fecal occult blood test was positive with no parasitosis. An abdominal tomography scan with contrast showed thickening of the gastric antrum, duodenum, and splenic flexure of the colon, with para-aortic and mesenteric adenomegaly and free intra-abdominal fluid (Fig. 1A and B). There were no thoracic alterations. Paracentesis was carried out, finding ascitic fluid with abundant inflammatory cellularity (Fig. 2A) (eosinophils 70%; neutrophils 10%; lymphocytes 3%; histiocytes 6%; plasmacytes 1%; and mesothelial cells 10%). There were no malignant cells, culture was negative, and the adenosine deaminase (ADA) level was 80 U/L. Esophagogastroduodenoscopy revealed very congestive gastroduodenal mucosa. Duodenal histopathology showed focal duodenal villous atrophy and countless eosinophils arranged in sheets, with glandular epithelium permeability (Fig. 2B). Colonoscopy was performed, taking random biopsies per segment, including the ileum. During the endoscopic study, no altered mucosa was identified, nor were there relevant findings in the reports. In other laboratory tests, IgE was found to be three-times above the upper limit of normal, accompanied by mild hypoalbuminemia. For the differential diagnosis, biochemical, hepatorenal, and electrolyte analyzes were carried out, along with tests for serum HIV, toxoplasma, vitamin B12, rheumatoid factor, antinuclear antibodies, antiDNA, ANCAs, serum tryptase, IgG, IgM, and IgA. All the results were within normal parameters. Flow cytometry in blood and immunohistochemistry of gastrointestinal tissues ruled out neoplastic involvement. The tuberculin skin test with PPD was nonreactive. The diagnostic conclusion was EGE and oral treatment with prednisone, 40 mg daily, was started. Symptoms resolved at 72 hours, abdominal distension decreased, and control for eosinophils in blood was normal at two weeks. The treatment was progressively suspended after eight weeks. The patient is currently asymptomatic with a normal eosinophil count and no abnormalities in the control contrast-enhanced magnetic resonance imaging of the abdomen and pelvis.
A) ascitic fluid cytology (eosinophils 70%; neutrophils 10%; lymphocytes 3%; histiocytes 6%; plasmacytes 1%; and mesothelial cells 10%), with no malignant cells. B) duodenal histopathology image showing focal villous atrophy and countless eosinophils, with glandular epithelium permeability.
EGE is a rule-out diagnosis.1 In the present case, infiltration by eosinophils into the three layers of the intestinal wall was inferred due to the presence of ascites, hypoalbuminemia, villous atrophy, and images of thickening of the gastrointestinal tract. Other differential diagnoses were ruled out through a meticulous analysis. ADA in the ascitic fluid, although positive, was below 100 U/L, with no histologic, immunologic, or clinical findings to suggest the diagnosis. The therapeutic response confirmed the diagnosis.
Until 2017, only 5 cases of postgestational EGE had been reported in the international literature, none of which involved three layers; one of the cases recurred in a second pregnancy.4 There has been an exponential increase of reports in the past five years, suggesting the possibility that pregnancy could act as a trigger. The predominant maternal immune response during pregnancy is humoral, which is why cell-mediated diseases, such as rheumatoid arthritis, improve during pregnancy, whereas others, such as systemic lupus erythematosus, worsen. This is consistent with a downregulated Th1-mediated immune response and an enhanced Th2-mediated response. Thus, it is possible that these changes during pregnancy caused the symptoms of postgestational EGE in the patient described herein.6
Ethical considerationsThe authors declare that this article contains no personal information that can identify the patient, preserving her anonymity according to institutional protocol. Informed consent was not requested for the publication of this case because no personal data or images are presented that could identify the patient. This article meets the current bioethical research regulations, and no experiments were conducted on animals or humans. The institutional ethics committee of the Hospital Universitario del Caribe in Cartagena, Colombia, authorized the present publication.
Financial disclosureNo financial support was received in relation to this article.
Conflict of interestThe authors declare that there is no conflict of interest.