Endoscopic submucosal dissection (ESD) in the treatment of superficial neoplasias of the gastrointestinal tract is currently one of the greatest advances in therapeutic endoscopy. Due to its high technical complexity, it is not yet a routine procedure in Latin America. The aim of the present study was to present the experience in Brazil with ESD in superficial gastric neoplasias, based on training received from Japanese experts.
Materials and methodsA retrospective study was conducted, in which information was prospectively collected from a database that included all patients that underwent ESD due to superficial gastric neoplasias at two endoscopy referral centers in Brazil, within the time frame of June 2008 to June 2019. En bloc, complete, and curative resection rates were calculated, along with the local recurrence rate and adverse events.
ResultsA total of 103 ESDs for superficial gastric neoplasias were performed during the study period. Eighty of those patients (77.6%) presented with early malignant gastric neoplasias or premalignant lesions (adenocarcinoma: 52.5%, high-grade dysplasia: 27.5%, low-grade dysplasia: 16.3%, and neuroendocrine tumors: 3.8%). Overall en bloc and complete resection rates for the superficial gastric neoplasias were 96.3% and 92.5%, respectively, whereas the curative resection rate based on expanded criteria was 76%.
ConclusionsESD for the treatment of superficial gastric neoplasias is a safe and effective therapeutic modality in Latin America, with results similar to those shown in the most representative Japanese studies.
La disección endoscópica de submucosa (ESD) en el tratamiento de las neoplasias superficiales del tracto gastrointestinal es uno de los más grandes avances en endoscopia terapéutica en la actualidad. En América Latina aún no se utiliza de manera rutinaria debido a la alta complejidad técnica. El objetivo de este estudio es presentar la experiencia con ESD en neoplasias gástricas superficiales desarrollada en Brasil a partir del entrenamiento con expertos japoneses.
Material y metodosEstudio retrospectivo de un banco de datos recolectado prospectivamente, que incluyo a todos los pacientes sometidos a ESD por neoplasias superficiales de estómago desde Junio del 2008 hasta Junio del 2019 en dos centros endoscópicos de referencia en Brasil. Calculando las tasas de resección en bloque, completa y curativa, así como también la tasa de recurrencia local y eventos adversos.
ResultadosEn el periodo descrito se incluyeron 103 procedimientos de ESD para neoplasias superficiales gástricas, de las cuales 80 (77.6%) fueron neoplasias gástricas malignas tempranas o lesiones premalignas (adenocarcinoma: 52.5%, displasia de alto grado: 27.5%, displasia de bajo grado: 16.3% y tumores neuroendocrinos: 3.8%). Las tasas globales de resección en bloque y completa para las neoplasias gástricas superficiales fueron del 96.3% y 92.5%, respectivamente, mientras que la tasa de resección curativa con base en los criterios expandidos fue del 76%.
ConclusionesLa ESD para el tratamiento de lesiones neoplásicas gástricas superficiales es una modalidad terapéutica efectiva y segura en Latinoamérica, presentando resultados similares a los mostrados en los estudios japoneses más representativos.
Gastric cancer continues to be a disease with high morbidity and mortality rates, especially in Latin American countries, becoming one of the first causes of cancer death worldwide, according to the World Health Organization (WHO).1–3 Standard treatment of gastric cancer is gastrectomy with lymph node dissection.1–8 In recent years, the development of endoscopic technology, including digital chromoendoscopy and optical magnification, has resulted in a significant increase of early stage gastric cancer detection, especially in the Asian countries of Japan and South Korea.
Likewise, the advances in therapeutic endoscopy have enabled the eradication of superficial gastric neoplasias with high safety and efficacy rates. Endoscopic mucosal resection was the first resection technique created for the eradication of superficial gastric lesions. However, endoscopic mucosal resection has limitations, such as not being able to carry out en bloc removal of lesions larger than 15 to 20mm, resulting in a high rate of local recurrence in those cases.9–11 Toward the end of the 1990s, endoscopic submucosal dissection (ESD) was developed in Japan, making en bloc endoscopic resection of superficial neoplasias possible, regardless of their size, and achieving higher curative resection rates and lower local recurrence rates. In addition, tumor removal in a single specimen enables more accurate histologic study and more reliable staging.9–15
ESD is currently one of the most innovative and revolutionary therapeutic procedures in medical practice but its incorporation outside of Japan has been slow and limited, especially in Latin America. The main limitations to its performance are its high technical complexity and the long learning curve required for its correct application, which has been more notorious in Western countries. Likewise, different research groups have shown that the ESD complication rate is similar to that of the endoscopic mucosal resection rate, when performed by experts.15–20 There are few Latin American studies that show the benefits of ESD in the treatment of early gastric cancer (EGC).16–20 The aim of the present study was to present the results of two endoscopic centers specializing in ESD in Brazil, based on formal training received in Japan, and compare them with the results obtained in Japanese endoscopy centers recognized worldwide.
Materials and methodsPatientsA retrospective study was conducted that included all patients that underwent ESD due to superficial gastric neoplasias (dysplasias, adenocarcinomas, and neuroendocrine tumors) at two endoscopy referral centers in Brazil, within the time frame of June 2008 and June 2019. Patients with submucosal neoplasias or advanced tumors were excluded. The study was approved by the ethics and review committees of the two institutions. The information was prospectively collected from a database and the following variables were analyzed: age, sex, type of gastric neoplastic lesion, preoperative biopsy, location, size, endoscopic Paris classification, procedure duration, final histologic report, adverse events, and hospital stay. The en bloc resection rate, complete resection rate with disease-free margins (R0 resection), and the curative resection rate according to current Japanese guidelines were also calculated.8,12 In addition, the local recurrence and metachronous lesion rates were analyzed in patients that accepted the recommendation of endoscopic follow-up.
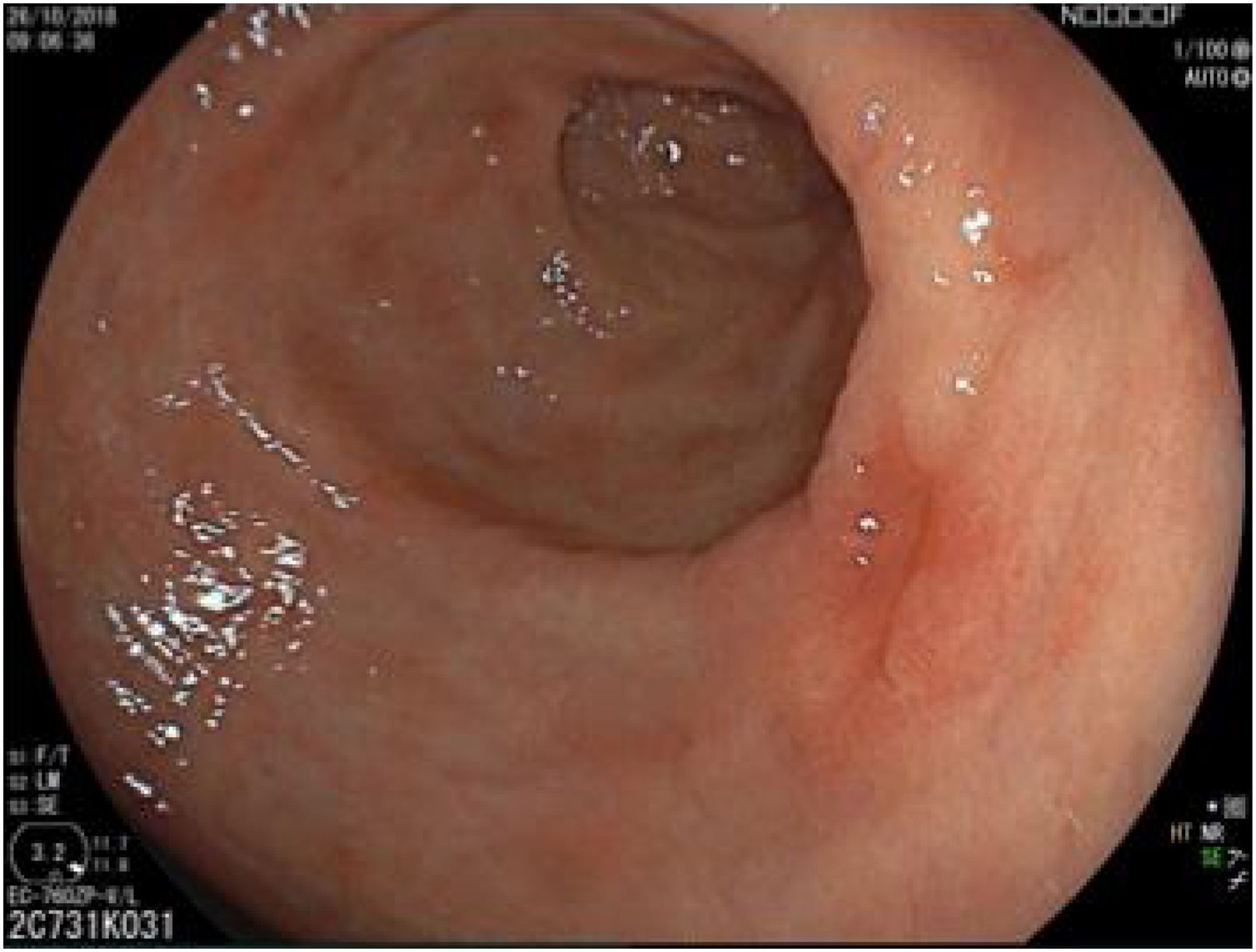
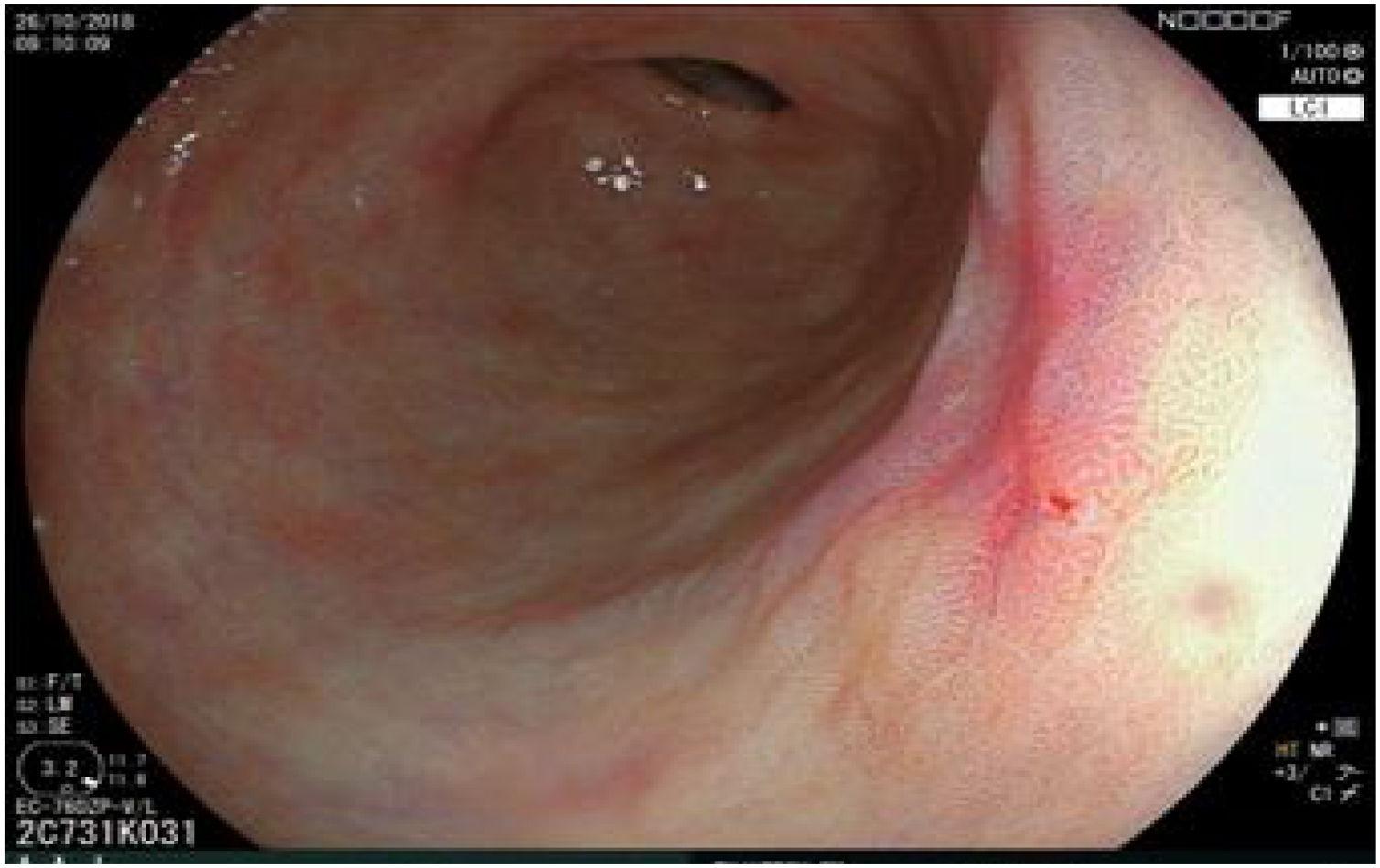
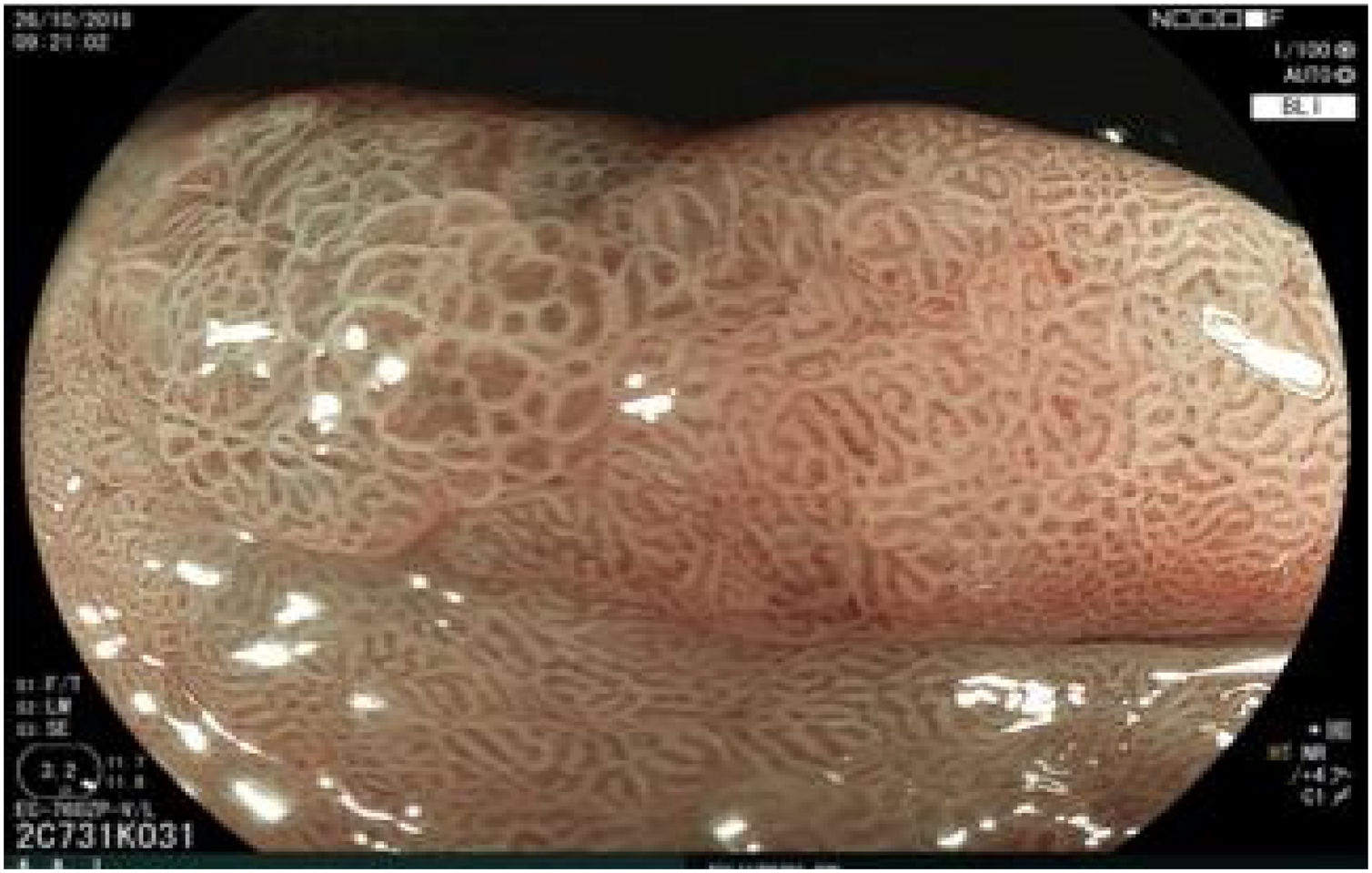
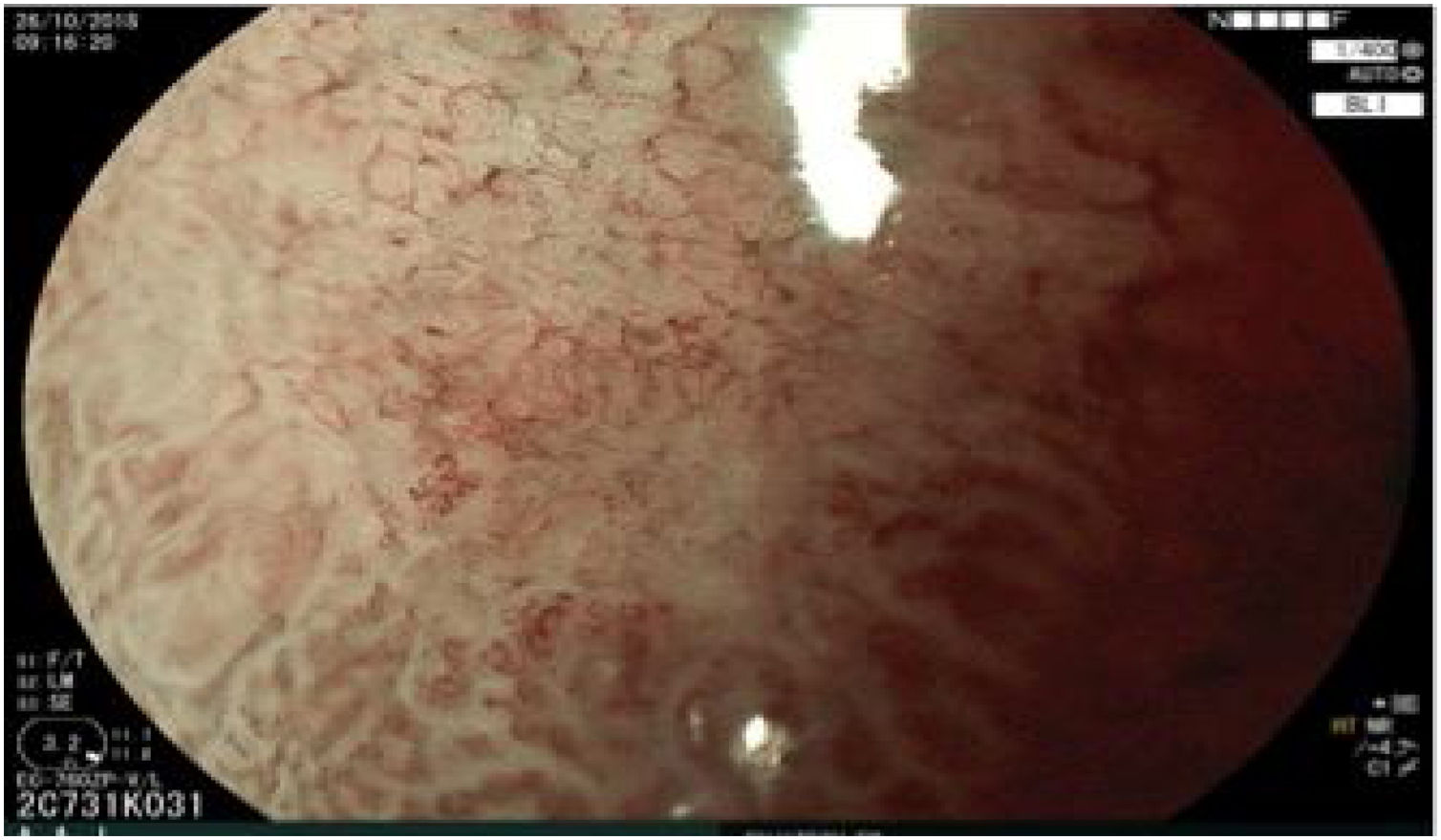
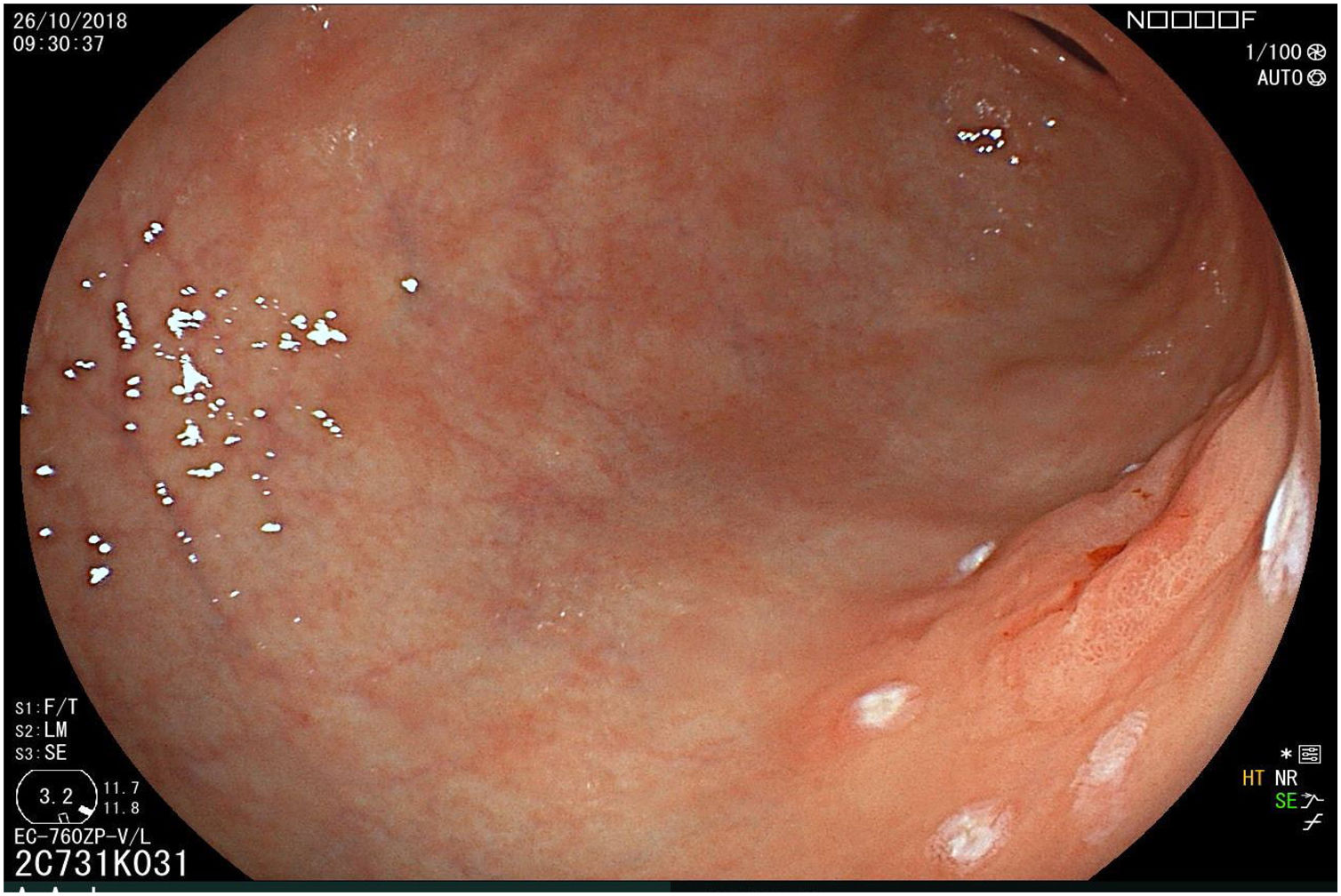
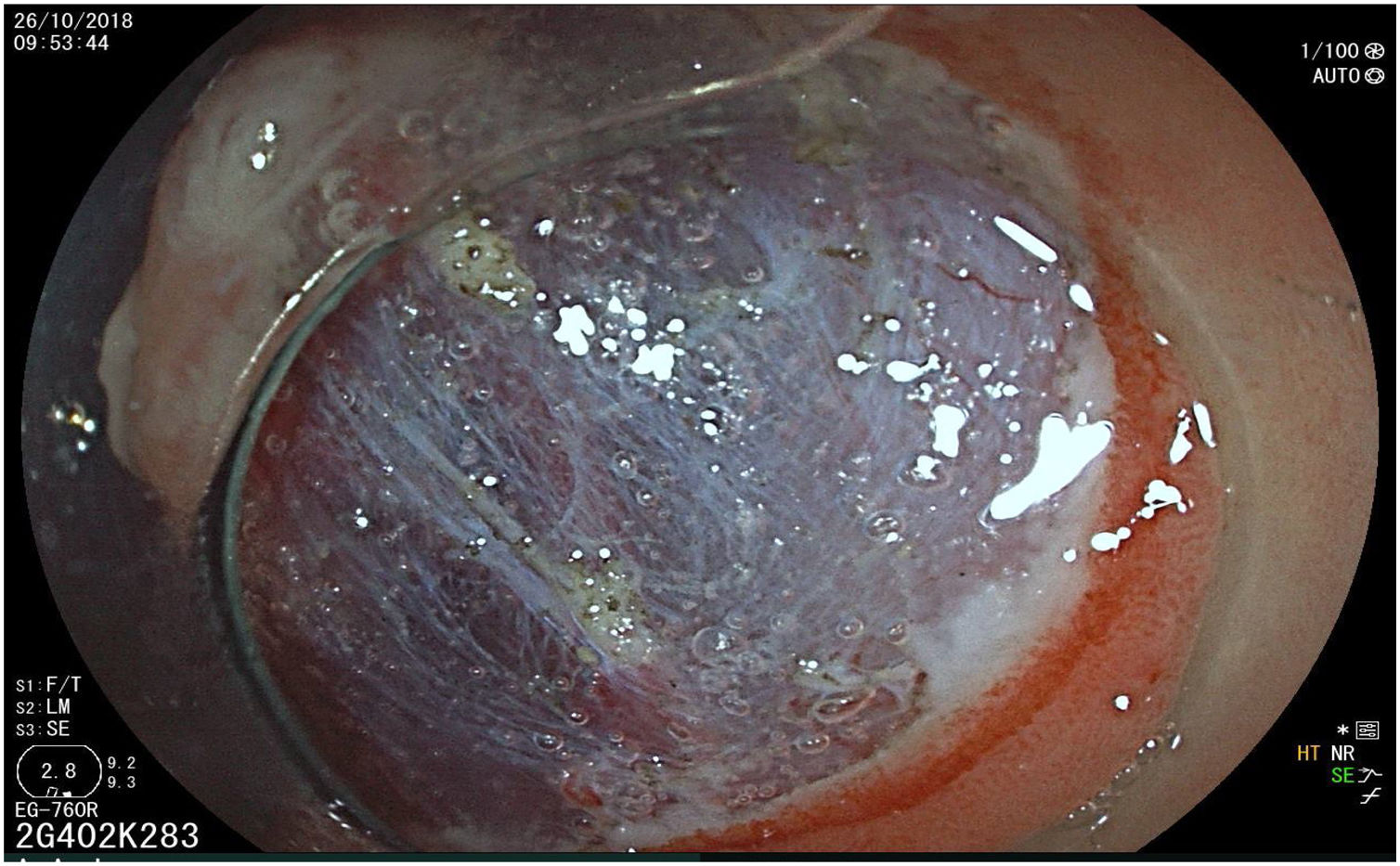
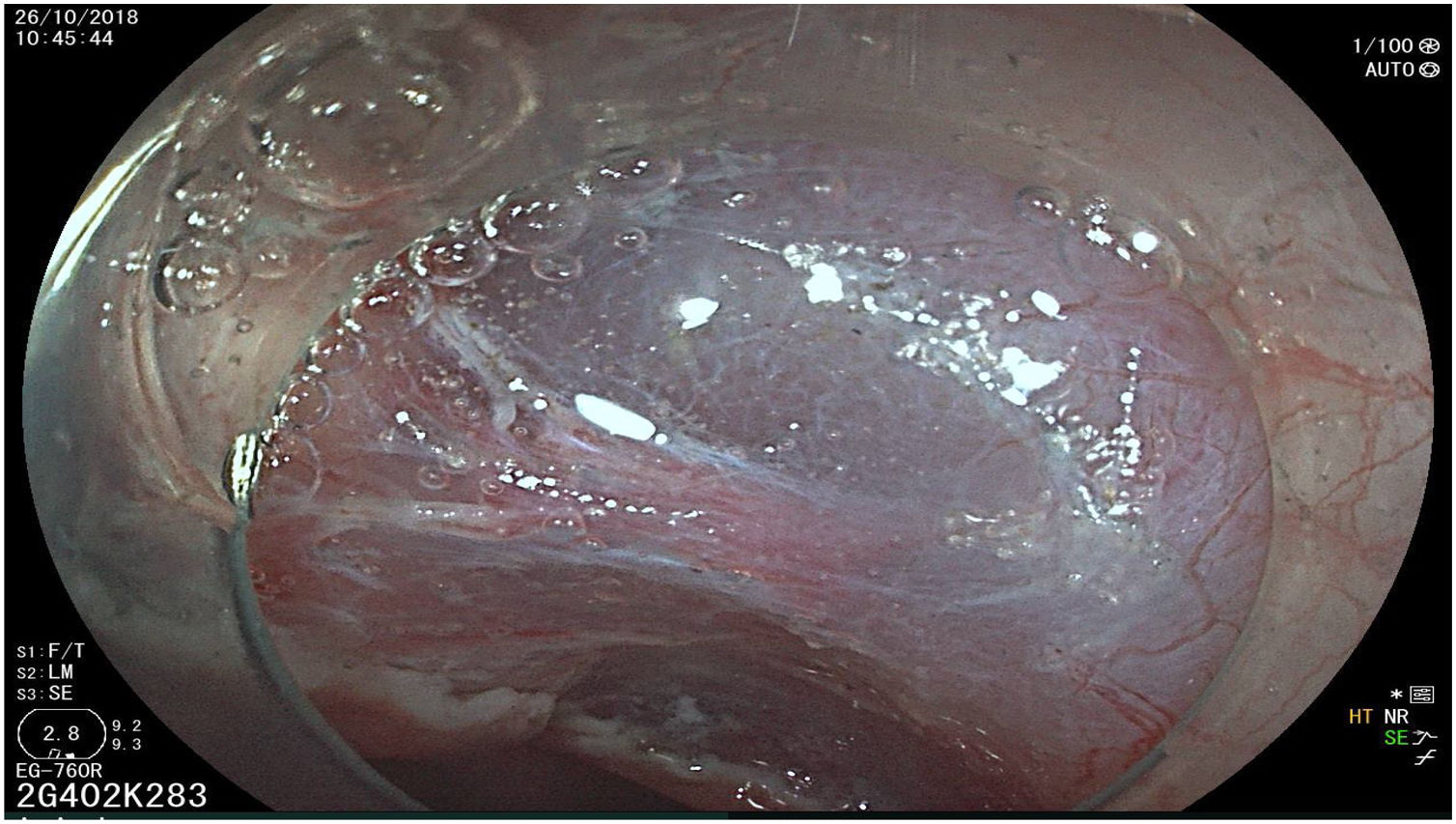
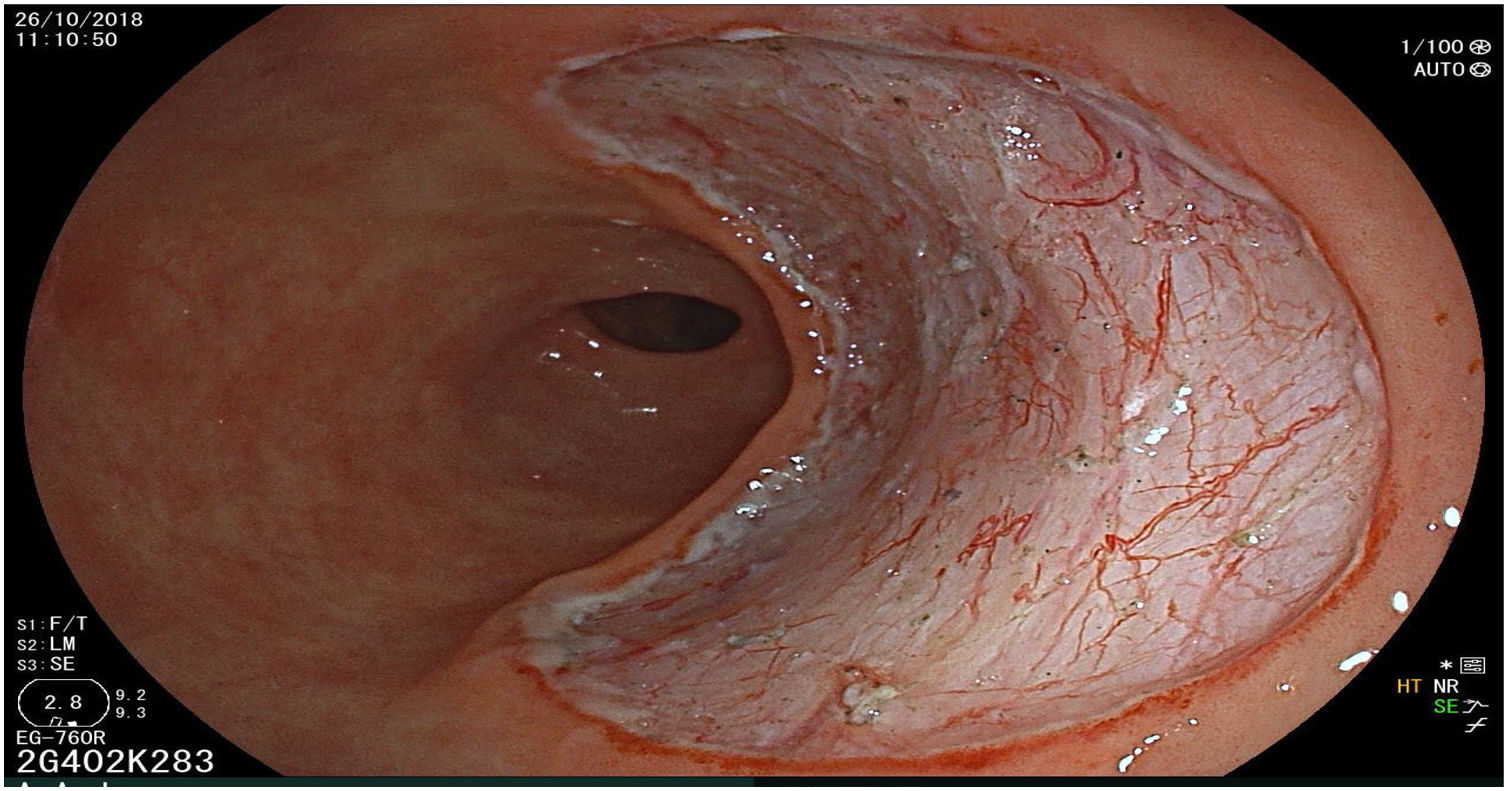
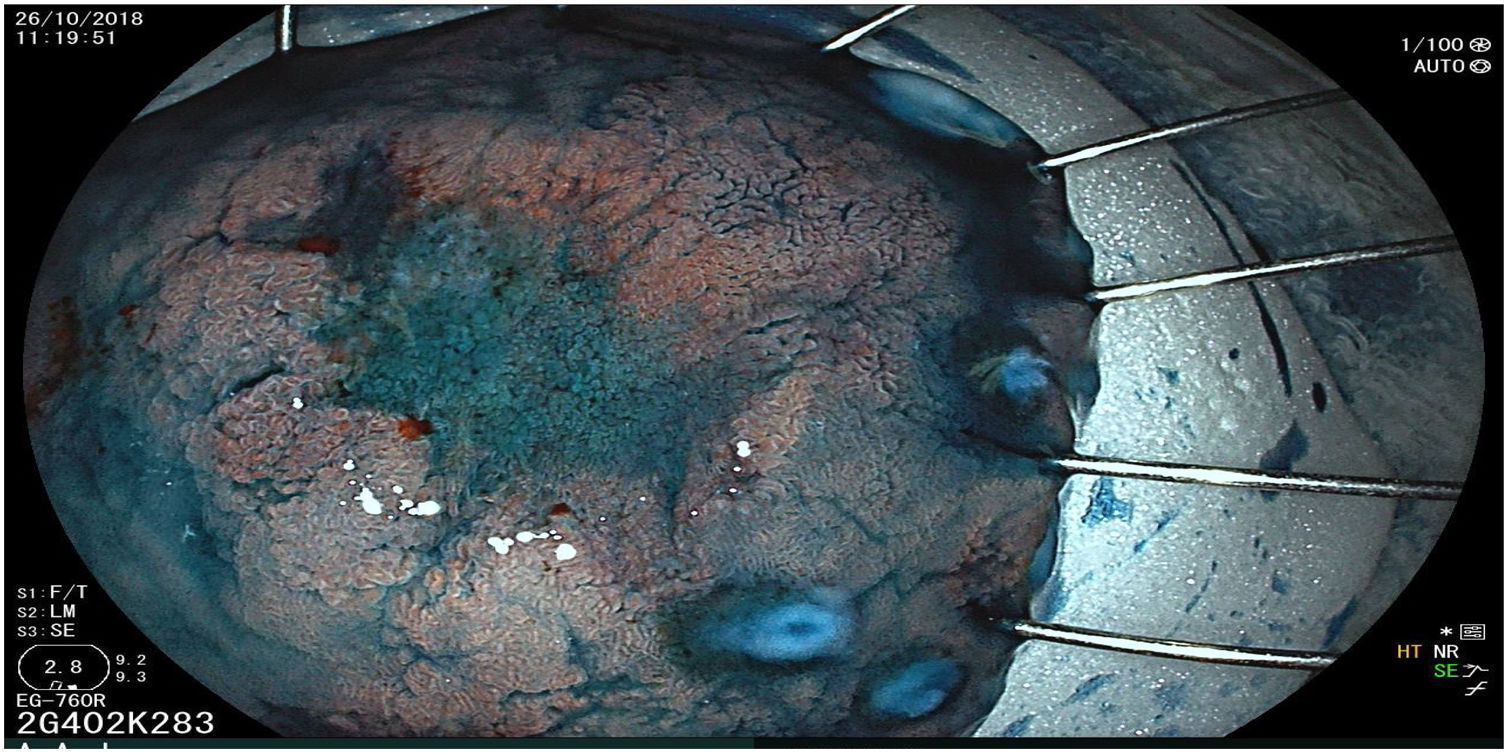
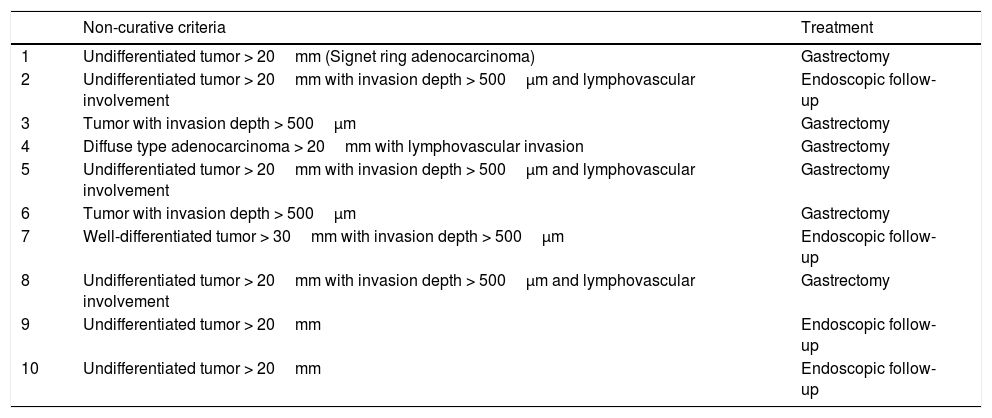
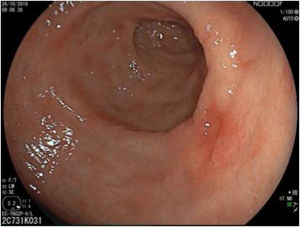
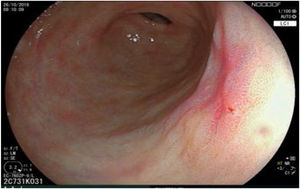
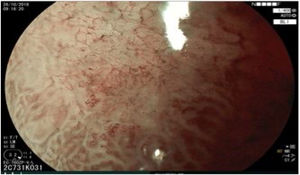
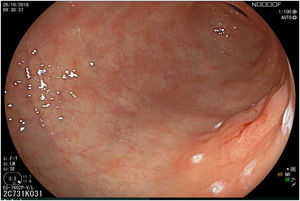
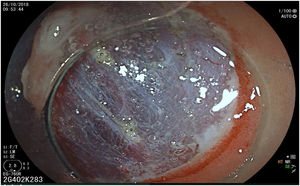
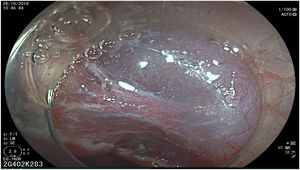
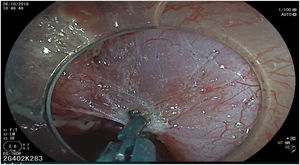
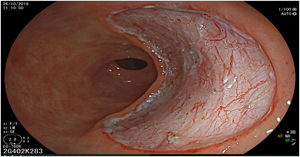
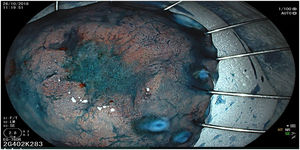
The endoscopic classification utilized to diagnose all lesions included in the present study was the Paris classification, whose validity is widely accepted by the different international consensuses and clinical guidelines. The following techniques were employed in the present study for the detection and characterization of the gastric lesions as follows: High-resolution white light endoscopy associated with a detailed and systematic review of the integrity of the gastric mucosa was initially utilized to identify lesions (Fig. 1). FICE (Flexible Spectral Imaging Colour Enhancement, Fujifilm Co., Japan) virtual chromoendoscopy or chromoendoscopy with 0.4% indigo carmine contrast staining were then employed to better characterize and delimit the lesions found (Fig. 2). In some cases, to optimize the evaluation of gastric abnormalities found (emphasizing special details, such as determining the microvascular pattern and specific alterations of the microsurface of the mucosa), minimum endoscopic image magnification, together with LCI (Linked Color Imaging, Fujifilm Co., Japan) and BLI (Blue Laser Imaging, Fujifilm Co., Japan) virtual chromoendoscopy, was utilized to show the demarcation line of the neoplastic lesion (Fig. 3), as well as microvascularization alterations (Fig. 4).
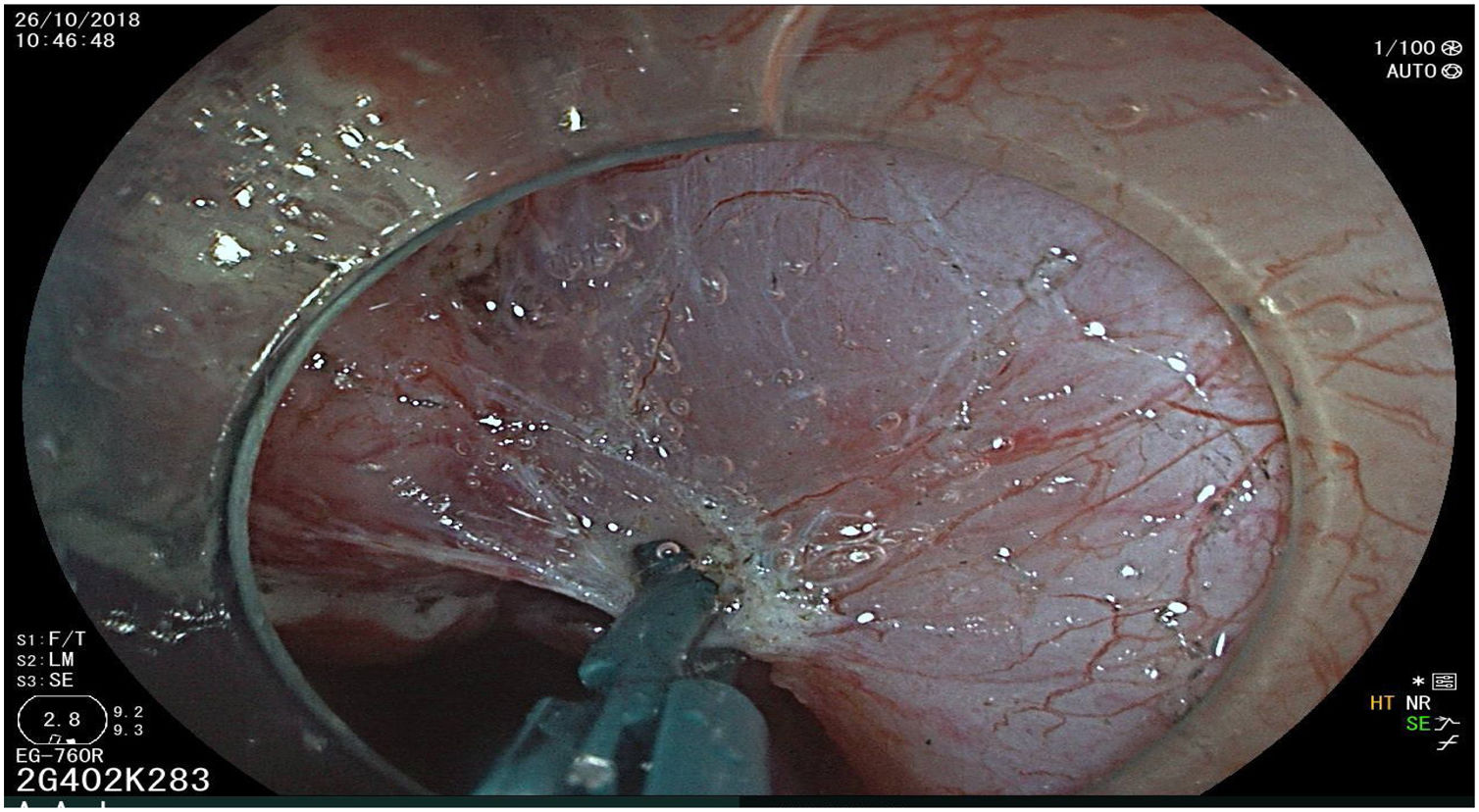
All the patients were treated by the same endoscopist (VA), who is one of the pioneers in ESD in Latin America. He received formal theoretic and practical training in Japan, visiting internationally recognized endoscopy centers, where he had numerous opportunities to assist experts in procedures on humans and perform ESD under supervision on animal models. In addition, he has participated as the lead researcher in different studies on ESD. Briefly, the ESD technique employed was the following: a therapeutic gastroscope with a 3.2mm working channel (450 RD, Fujifilm Co., Japan), the 2.0 or 2.5 BT Flush Knife (Fujifilm Co., Japan) connected to the electrosurgical unit (ERBE VIO 200S, 200D, or 300D, Tubingen, Germany), and a 4mm long cap on the tip of the equipment (Top Co., Japan) were utilized to for better visualization in the dissection field. The following steps were employed: Demarcation, Soft coagulation mode, Effect 6, 100W; Mucosal incision: Endocut I, Effect 2, Cut length 3, Cut interval 2; Submucosal dissection: Forced coagulation mode, Effect 3, 50W; Pre-hemostasis of the vessels: Soft coagulation mode, Effect 6, 100W. Vessel hemostasis was carried out using the same Flush Knife and the coagulation grasper was used for larger vessels (Coag Grasper, Olympus Co., Japan). In 75.7% of the endoscopic dissections performed, the teardrop presentation of sodium hyaluronate at 0.4% (Adaptis Fresh®, Legrand Laboratory, Brazil) was the substance used for submucosal injection at a mean solution volume of 10cm3 per patient and at an additional cost of 10 USD per procedure. Other substances used were mannitol (9.7% of the cases), hydroxypropyl methylcellulose (8.7%), and saline solution (3.8%). Figs. 1–10 show the technique employed at each step of the procedure in the present study.
5cm en bloc ESD specimen, fixed and pulled back with pins. Observe the depressed zone with 0.4% indigo carmine contrast staining. Histology showed well-differentiated intestinal-type adenocarcinoma with invasion into the lamina propria associated with high-grade and low-grade dysplasia. Disease-free deep lateral margins with no vascular or lymphatic invasion. R0 resection with curative criteria.
Data tabulation was performed utilizing the Microsoft Excel program for Windows 2010 and the statistical analysis was carried out with the STATA version 15 program. Sample size was calculated, accepting an alpha risk of 0.95 for a specification of ±0.032 units in a bilateral contrast for an estimated proportion of 0.9, specifying a random population sample of 80 subjects (n=80), assuming that the population was 103 participants and calculating a 2% replacement rate.
Ethical considerationsThe authors declare that during the present research no experiments were performed on animals or humans. It was a description of gastric ESD cohorts from 2 endoscopy referral centers in Brazil, for which informed consent was obtained. The study was approved by the institutional review committee and meets all the established regulations for scientific research, including data confidentiality for each of the patients enrolled in the analysis.
ResultsA total of 103 gastric ESDs were performed during the study period. Twenty-three cases were excluded for the following reasons: 12 submucosal neoplasias (GIST: 6, ectopic pancreas: 3, submucosal fibromas: 2, and Vanek’s tumor: 1), 6 polypoid lesions (hyperplastic polyps: 3, fibrovascular polyp: 1, fundic gland polyp: 1, and inflammatory fibroid polyp:1), 3 intestinal metaplastic lesions, and 2 advanced tumors with no lifting sign.
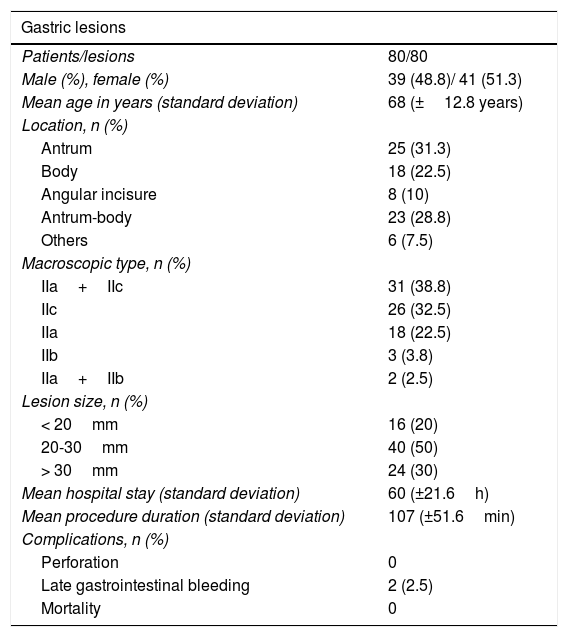
Of the 80 patients analyzed, 41 were women (51.3%) and 39 were men (48.8%). Mean patient age was 68 years (standard deviation: 12.8 years), mean lesion size was 28mm (standard deviation: 11.2mm), and mean procedure duration was 107min (standard deviation: 51.6min). The most frequent lesion locations were the antrum in 25 (31.3%) cases and the body in 18 (22.5%). Tables 1 and 2 show the clinical and pathologic characteristics of the study patients.
Clinical and pathologic characteristics of patients and lesions.
| Gastric lesions | |
|---|---|
| Patients/lesions | 80/80 |
| Male (%), female (%) | 39 (48.8)/ 41 (51.3) |
| Mean age in years (standard deviation) | 68 (±12.8 years) |
| Location, n (%) | |
| Antrum | 25 (31.3) |
| Body | 18 (22.5) |
| Angular incisure | 8 (10) |
| Antrum-body | 23 (28.8) |
| Others | 6 (7.5) |
| Macroscopic type, n (%) | |
| IIa+IIc | 31 (38.8) |
| IIc | 26 (32.5) |
| IIa | 18 (22.5) |
| IIb | 3 (3.8) |
| IIa+IIb | 2 (2.5) |
| Lesion size, n (%) | |
| < 20mm | 16 (20) |
| 20-30mm | 40 (50) |
| > 30mm | 24 (30) |
| Mean hospital stay (standard deviation) | 60 (±21.6h) |
| Mean procedure duration (standard deviation) | 107 (±51.6min) |
| Complications, n (%) | |
| Perforation | 0 |
| Late gastrointestinal bleeding | 2 (2.5) |
| Mortality | 0 |
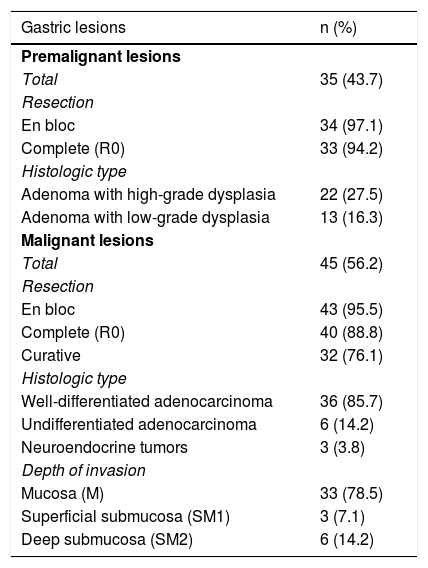
Histopathologic characteristics of the patients.
| Gastric lesions | n (%) |
|---|---|
| Premalignant lesions | |
| Total | 35 (43.7) |
| Resection | |
| En bloc | 34 (97.1) |
| Complete (R0) | 33 (94.2) |
| Histologic type | |
| Adenoma with high-grade dysplasia | 22 (27.5) |
| Adenoma with low-grade dysplasia | 13 (16.3) |
| Malignant lesions | |
| Total | 45 (56.2) |
| Resection | |
| En bloc | 43 (95.5) |
| Complete (R0) | 40 (88.8) |
| Curative | 32 (76.1) |
| Histologic type | |
| Well-differentiated adenocarcinoma | 36 (85.7) |
| Undifferentiated adenocarcinoma | 6 (14.2) |
| Neuroendocrine tumors | 3 (3.8) |
| Depth of invasion | |
| Mucosa (M) | 33 (78.5) |
| Superficial submucosa (SM1) | 3 (7.1) |
| Deep submucosa (SM2) | 6 (14.2) |
Of the 80 resected lesions, 42 (52.5%) were adenocarcinomas, 22 (27.5%) were low-grade dysplasias, 13 (16.3%) were high-grade dysplasias, and 3 (3.8%) were neuroendocrine tumors. With respect to macroscopic morphology, 31 (38.8%) lesions were described as IIa+IIc, 26 (32.5%) as IIc, 18 (22.5%) as IIa, 3 (3.8%) as IIb, and 2 (2.5%) as IIa+IIb. The overall en bloc resection rate achieved was 96.3%, of which 97.1% (34/35) were premalignant lesions and 95.5% (43/45) were malignant lesions. Three cases required hybrid resection. The overall complete resection (R0) rate was 92.5%, of which 94.2% were premalignant lesions and 88.8% were malignant lesions.
Of the patients diagnosed with adenocarcinoma (42 patients), curative resections based on expanded criteria were obtained in 32/42 patients (76%) with gastric adenocarcinoma. Regarding invasion depth, 6 patients had SM2 submucosal invasion and 3 had SM1 submucosal invasion. The remaining 33 patients were diagnosed with adenocarcinoma limited to the mucosa. There was a histopathologic discrepancy between pre-resection endoscopic biopsy and the specimen resected through ESD in 30/80 cases (37.5%).
Mean post-procedure hospital stay was 60 h (standard deviation±21.6h). With respect to complications, only 2 cases presented with post-ESD gastrointestinal bleeding (6h and 24h after the procedure). They were managed endoscopically through coagulation with a monopolar coagulation grasper (Coag Grasper, Olympus Co., Japan) (Soft mode, 80 W, Effect 4) and the application of hemostatic clips, with favorable progression. There were no deaths (mortality 0%) or perforations. Ten cases (23.8%) were considered non-curative, of which 6 (7.5%) presented with lymphovascular invasion. Sixty percent (6/10) of the non-curative ESD cases underwent gastrectomy and one of those cases presented with signet cell adenocarcinoma. The other 40% (4/10) only underwent close endoscopic evaluation because they were older adults above 70 years of age, with important comorbidities. In addition, the tumor site in 3 of them was at the level of the cardia and proximal body, which is the indication for total gastrectomy - a surgery with a high postoperative risk for morbidity and mortality. However, there were no signs of residual lesion in any of them, up to the mean follow-up time of 22.5 months (Table 3). Finally, all the study patients had endoscopic follow-up. It was carried out over a mean period of 17 months (standard deviation±14.5 months) in 49 patients, finding only 3 cases of metachronous lesions (3.7%) and one case of local recurrence (1.2%). The case with local recurrence was a young female patient with an undifferentiated tumor, who was referred for gastrectomy.
Characteristics of non-curative resected lesions.
| Non-curative criteria | Treatment | |
|---|---|---|
| 1 | Undifferentiated tumor > 20mm (Signet ring adenocarcinoma) | Gastrectomy |
| 2 | Undifferentiated tumor > 20mm with invasion depth > 500μm and lymphovascular involvement | Endoscopic follow-up |
| 3 | Tumor with invasion depth > 500μm | Gastrectomy |
| 4 | Diffuse type adenocarcinoma > 20mm with lymphovascular invasion | Gastrectomy |
| 5 | Undifferentiated tumor > 20mm with invasion depth > 500μm and lymphovascular involvement | Gastrectomy |
| 6 | Tumor with invasion depth > 500μm | Gastrectomy |
| 7 | Well-differentiated tumor > 30mm with invasion depth > 500μm | Endoscopic follow-up |
| 8 | Undifferentiated tumor > 20mm with invasion depth > 500μm and lymphovascular involvement | Gastrectomy |
| 9 | Undifferentiated tumor > 20mm | Endoscopic follow-up |
| 10 | Undifferentiated tumor > 20mm | Endoscopic follow-up |
The detection and efficacious treatment of incipient gastric cancer is one of the primary challenges in modern gastroenterology worldwide, which is why different research groups have attempted to standardize concepts in the last few years. The most recent and updated Latin American consensus was formulated by Icaza-Chávez et al.1 in Mexico. Seventeen experts on the theme came together and carried out a thorough search in the main medical databases on articles published from January 1, 2008, to March 31, 2018, resulting in a consensus with 38 recommendations. Those recommendations established the current standards in early endoscopic diagnosis, risk stratification for premalignant lesions, and the most adequate therapeutic approach, emphasizing the importance and efficacy of endoscopic resection, mainly ESD, for the treatment of premalignant lesions and early malignant lesions.
Thus, we consider research such as ours opportune, as it shows the Western experience in ESD, especially in Latin America, which serves to reinforce those consensuses and motivate formal training of the procedure for its correct application in different parts of the world.
For many years, gastrectomy has been the standard treatment for EGC, with optimum results in relation to local recurrence. However, the high postoperative morbidity rate and frequent postoperative complications spurred the creation of new, less invasive techniques for the resection of those types of lesions.2,3 A recent comparative meta-analysis conducted by Li et al.,4 that included 14 studies with 5,112 patients, showed that ESD, in the treatment of EGC, had advantages over conventional gastrectomy, with 1) lower postoperative complication rates, with a weighted OR of 0.39, 95% CI, and p<0.001, supporting the low post-ESD complication rate found in our study (2.5%); 2) lower procedure times with statistically significant results and a mean weighted difference of 140min, also coinciding with the short time calculated in our analysis (107min.); and 3) shorter hospital stay, showing a mean weighted difference of 5.41 days (95% CI, p<0.001), concurring with our result that had an even lower mean value (2.5 days) than the hospital stay calculated in the meta-analysis. However, that study found a higher tumor recurrence rate for ESD, with an OR of 9.24 (5.94-14.36) and a p<0.001, but no statistically significant differences in long-term disease-free survival between the two groups. Importantly, by using ESD, instead of gastrectomy, to treat EGC, the affected organ is spared. In a majority of cases, said organ already presents with pre-neoplastic conditions associated with the main resected lesion, such as intestinal metaplasia and gastric atrophy. Thus, the recurrent and/or metachronous lesion rates are expected to be higher in groups that undergo endoscopic resection. Despite that evidence, our study found only one case of local recurrence (1.2%) during the mean 17-month follow-up period, supporting ESD in that aspect. Even though our recurrence results do not concur with those of the abovementioned study, they do coincide with those of the majority of Japanese research groups.
Gonzalez et al.5 recently described a survey on the status of ESD in Latin America. Those authors found that ESD was presently performed on humans at only 23 endoscopy centers, located in 10 out of 23 countries (43%). Interestingly, that study showed that only 10 endoscopists (40%), from a total of 25 Latin American ESD operators, had published an article related to their experience with ESD. Given that setting, we consider it very important to provide an in-depth report on the experience with ESD developed at Latin American referral centers, to promote and promulgate the method, especially among young endoscopists, whose basic requirements include having previous experience in endoscopic mucosal resection and the management of its complications, as well as having training in advanced diagnostic endoscopy techniques (chromoendoscopy+magnifying endoscopy), for the detection and characterization of early lesions. Studies such as ours enable the analysis of results in Latin American countries and their comparison with major experiences worldwide, such as the expert centers in Japan. With those motivations and justifications, we developed the present retrospective study, analyzing our data on the performance of gastric ESD over an 11-year period.
We have described herein the experience in gastric ESD at two endoscopy referral centers in Brazil, following the formal theoretic and practical training the operator received in Japan, where he had the opportunity to perform ESD on animal models under the supervision of world experts. After having carried out over one hundred gastric ESDs, we have demonstrated en bloc and R0 resection rates above 90%, with a very low rate of adverse events (under 3%) and local recurrence (1.2%). A total of 32/42 ESDs were considered curative (based on expanded criteria). One of the biggest advantages of ESD is that it enables a more reliable histologic analysis, which makes it possible to indicate gastrectomy exclusively for those patients whose histopathologic findings show a high risk for lymphatic metastasis, thus preventing unnecessary surgeries. We observed a histopathologic discrepancy in our study between the endoscopic pre-resection biopsy and the histologic analysis of the specimen resected by ESD, in nearly one-third of the cases (37.5%). That finding has also been described in other, mainly Japanese, ESD case series, reflecting the need for Western studies on the theme, to have a better understanding of said discrepancy.
Different research results have shown the efficacy of ESD in the treatment of EGC, including the expanded criteria proposed by the clinical guidelines of the European Society of Gastrointestinal Endoscopy (ESGE).6–13 Takagi et al.14 performed 1,559 ESDs in EGC, finding 145 histopathologically confirmed cases with superficial submucosal invasion (< 500μm) that were divided into 2 groups: one group underwent radical surgery, and one group had no additional treatment. A survival analysis was then conducted on each group. There were no statistically significant differences between the two groups, regarding 3 and 5-year survival. The local recurrence rate was 2.6% for the radical surgery group, and 1.4% for the group that only had endoscopic follow-up, showing, as in our study, that ESD in expert hands is highly efficacious in the treatment of EGC, even when expanded criteria are taken into account.
Experience with ESD in the treatment of superficial gastric neoplasias is still limited in Western countries due to its high technical complexity and long learning curve needed for its correct application.15–17 According to the consensus of world experts, the performance of safe and effective gastric ESD requires having performed a minimum of 40 ESDs on humans, under the supervision of an experienced endoscopist, thus surpassing the initial phase of the long learning curve, as well as following the training algorithms proposed in Japanese specialized training program models.18
In a Western cohort, Tate et al.19 demonstrated the safety and efficacy of ESD in EGC in 121 patients (135 lesions), with en bloc, complete (R0), and curative (considering expanded criteria) resection rates of 94.8%, 86.7%, and 79.2%, respectively, with a low complication rate (bleeding 5.2% and perforation 1.5%) and only 2 cases of local recurrence, during 71 months of follow-up. Thus, we can see, in a Western context, that the safety and efficacy of ESD is the same as in the procedures performed at the Japanese referral centers. Another Latin American study showing the benefits of ESD in EGC was conducted by Palacios et al.20 Published as an abstract, it reported that the study prospectively included 94 patients (105 lesions), over a 6-year period, resulting in en bloc, complete (R0), and curative resection rates of 99%, 98%, and 92%, respectively, with a low complication rate (7 cases of perforation [6.7%] and 4 cases of bleeding [3.8%]), a local recurrence rate of 1.4%, and 4 cases of metachronous lesions (6%). As in our analysis, those two studies show that, in Western countries, despite the current lack of experience and much lower number of resected cases that utilize ESD in superficial gastric lesions in Latin American centers, the results are similar to those of the most important studies conducted at Japanese and Korean centers. It should be clarified that the number of cases included in our study, despite the long study period, is mainly a result of the lack of a national early detection program for gastric neoplasias in Brazil, resulting in late diagnosis in the majority of patients, and consequently, not meeting the criteria for ESD. Unfortunately, that is the situation in the majority of Latin American countries.
Few studies analyze the impact of ESD on the treatment of the different types of superficial gastric neoplastic lesions, and the majority focus on dysplasias and early malignant neoplasias.21–24 Costa et al.25 performed 114 ESDs on gastric epithelial lesions (dysplasias and neoplasias), with the following findings in the post-resection specimen: low-grade dysplasia in 37.5%; high-grade dysplasia in 34.8%; intramucosal adenocarcinoma in 13.4%; submucosal adenocarcinoma in 10.7%, and lesions with negative histology for neoplastic dysplasia in 3.6%, as well as en bloc, complete, and curative resection rates of 96.5%, 87.6%, and 83.2% respectively, a complication rate of 13.2% (bleeding 10.5% and perforation 0.9%), and 6 cases of local recurrence during 12 months of follow-up. We had similar results in our study, that also included subepithelial lesion involvement with high oncogenic potential (neuroendocrine tumors), resulting in one of the first positive Western experiences with ESD in the management of superficial gastric neoplasias.
High-resolution endoscopy (chromoendoscopy - magnifying endoscopy) and directed biopsy are essential for the endoscopic follow-up of gastric neoplastic lesions treated with ESD, primarily for the correct evaluation of post-resection cicatrization, looking for residual lesion, local recurrence, and metachronous lesions. Arantes et al.26 conducted a multicenter study at different endoscopy referral centers worldwide that evaluated the overall incidence and oncogenic potential of the post-ESD nodule-polypoid cicatrices of the superficial gastric neoplastic lesions that underwent en bloc, complete, and histologically confirmed curative resection. General incidence was calculated at 2.87% (22/766), located predominantly in the antral region (21/22), with no cases of local malignant neoplastic recurrence, during a mean follow-up period of 43 months. Those results show us that an adequately performed ESD for EGC reduces the local recurrence rate, regardless of the endoscopic aspect of the post-ESD cicatrix. However, endoscopic follow-up with directed biopsy is necessary in all patients that undergo ESD for EGC, with greater emphasis on those that present with expanded criteria for resection, as was done in our patient cohort.
One of the main limitations for performing ESD in EGC in Western countries is the potential risk for complications. Nevertheless, different studies have shown that when performed by expert endoscopists, the complication rate is extremely low and comparable to that of less complex procedures, such as endoscopic mucosal resection. Gastric perforation a major complication of ESD.27–30 Huh et al.31 reported a low rate of said complication in 556 patients that underwent ESD for treating EGC, finding 34 cases (6.1%) of post-ESD perforation. The majority were treated conservatively (endoscopic management in 27 patients and medical-conservative management in 6), with no statistically significant difference in long-term survival or local recurrence due to that complication, compared with the control group. Those results concur with ours, but in a different population.
ConclusionIn conclusion, ESD is currently considered the first-line treatment for superficial gastric neoplastic or dysplastic lesions, with excellent medium-term and long-term results (en bloc, complete, curative, and local recurrence rates) and an extremely low complication rate, when performed by experienced endoscopists. Therefore, the divulgence of new concepts in the management of early gastric cancer, based on expanded criteria, with special emphasis on the ESD technique, is essential. The creation of training centers for high complexity procedures is also necessary in countries where ESD performance is still limited, especially in Latin America, so that its beneficial effects can be achieved in our patients.
Financial disclosureNo financial support was received in relation to this study/article.
Conflict of interestThe authors declare that there is no conflict of interest.
Please cite this article as: Arantes V, Aliaga Ramos J, Pedrosa MS. Disección endoscópica de submucosa para neoplasias gástricas superficiales en dos hospitales de referencia en Brasil: ¿se pueden igualar los resultados de Japón y Corea del Sur? Revista de Gastroenterología de México. 2021;86:244–252.