Primary liver cancer is a public health problem in Mexico and the world. Liver transplantation (LT) is the ideal treatment for early hepatocellular carcinoma (HCC). Our aim was to evaluate the characteristics of patients with HCC and cholangiocarcinoma (CC) at two centers and identify transplantation candidates.
Materials and methodsA retrospective observational study was conducted at the Hepatology Center (HC) and the University Center Against Cancer (UCAC), within the time frame of 2012–2018. HCC or intrahepatic CC was confirmed in 109 patients. Staging classifications, transplant selection models, and a predictive model for post-LT recurrence were applied to the HCC patients.
ResultsOf the total population, 93% (n=102) presented with cirrhosis, 86% (n=94) had HCC (HC: 58%, UCAC: 42%), and 14% (n=15) had intrahepatic CC (HC: 40%, UCAC: 60%). Of the HC patients with HCC, Okuda I–II, BCLC A–B, and AFP levels <100ng/m predominated, whereas Okuda II-III, BCLC C-D, and AFP levels >1000ng/mL predominated in the UCAC patients. Half of the HC population with HCC met the criteria for LT, in contrast to 23% of the UCAC patients. Fifteen patients were evaluated for LT, and at present, six have undergone transplantation.
ConclusionsThe most frequent primary liver tumor was HCC. Patients from the HC presented with earlier-stage disease and a high number of them met the criteria for LT. Only patients from the HC underwent transplantation.
El cáncer primario de hígado es un problema de salud pública en México y en el mundo. El trasplante hepático (TH) es el tratamiento ideal para el carcinoma hepatocelular (CHC) temprano. El objetivo fue evaluar las características de los pacientes con CHC y colangiocarcinoma (CC) en dos centros e identificar a los candidatos a trasplante.
Material y métodosEstudio retrospectivo, observacional del 2012 al 2018 en el Centro de Hepatología (CH) y el Centro Universitario contra el Cáncer (CUCC). Se confirmó CHC o colangiocarcinoma intrahepático (CCi) en 109 pacientes, a los CHC se les aplicaron clasificaciones de estadiaje, modelos de selección para trasplante y modelo pronóstico de recidiva post-TH.
ResultadosDe la población total, 93% (n=102) eran cirróticos. El 86% (n=94) tuvo CHC (58% CH y 42% CUCC) y 14% (n=15) CCi (40% CH y 60% CUCC). En los pacientes con CHC del CH predominó Okuda I–II, clasificación de Barcelona Clinic Liver Cancer (BCLC) A–B y niveles <100ng/mL de alfafetoproteína (AFP), mientras que en el CUCC predominó Okuda II-III, BCLC C-D y niveles >1000ng/mL de AFP. La mitad de la población del CH con CHC cumplía con los criterios para TH, en cambio, en el CUCC solo lo hizo el 23%. Se valoraron 15 pacientes para TH y, a la fecha, se trasplantaron seis.
ConclusionesLa neoplasia primaria de hígado más frecuente fue CHC. Los pacientes del CH presentaron la enfermedad más temprana y una proporción más alta cumplía con los criterios para TH. Solo quienes pertenecían a este centro recibieron un trasplante.
Primary liver tumors are a worldwide public health problem, according to the World Health Organization (WHO). In 2018, a total of 841,808 new cases were registered. They are the sixth cause of cancer and hold fourth place in cancer death across the globe1. In Mexico, they have been reported to hold third place among cancer-related deaths2. Hepatocellular carcinoma (HCC) is the most frequent primary liver cancer3, followed by cholangiocarcinoma (CC)4. Approximately 90% of the cases of HCC develop in patients with cirrhosis and its most frequent etiologies are chronic hepatitis B virus (HBV) infection, chronic hepatitis C virus (HCV) infection, alcoholic liver disease (ALD), and in recent years, nonalcoholic steatohepatitis (NASH). Other less frequent causes of HCC are autoimmune liver diseases, hemochromatosis, exposure to aflatoxins, and Wilson’s disease3,5, among others.
CC is an aggressive tumor that develops from the epithelium of the biliary tract and is divided into intrahepatic CC (iCC) and extrahepatic CC (eCC). In turn, eCC is divided into hilar/perihilar CC (Klatskin tumor) and distal disease6. A positive association between CC has been found with cirrhosis of the liver, HBV infection, HCV infection, primary sclerosing cholangitis, choledocholithiasis, obesity, type 2 diabetes mellitus (DM2), and smoking7–8. The combined HCC-CC tumor is a rare neoplasia that accounts for <5% of all primary liver cancers9. In imaging studies, HCC-CC is almost indistinguishable from HCC, and even biopsy results often describe only one of the components10 Thus, diagnosis is difficult and is often confirmed after liver transplantation (LT), in the histopathologic study of the explant11.
According to international guidelines3,5, therapeutic management of HCC is divided into curative and noncurative. Surgical resection, radiofrequency ablation (RFA), cryotherapy, percutaneous injection of ethanol, and LT are considered curative treatments for HCC, given their long-term effectiveness. Currently available noncurative treatments are transcatheter arterial chemoembolization (TACE), radioembolization, and systemic therapy with sorafenib, which delay tumor progression and increase patient survival5,12. CC can be treated through surgical resection, RFA, and TACE, and systemic chemotherapy (CT) is opted for, in advanced stages of the disease13.
At present, LT is the ideal treatment for HCC because both the tumor and the cirrhotic liver are removed14. Recent studies have reported that up to 30% of all LTs in Europe and the United States are performed in patients with HCC3,15. Even though LT is considered the best treatment, not all patients are candidates for it. The Milan criteria are the worldwide gold standard for selecting the best candidates for LT in patients with early HCC16. Patients that meet those criteria have a 5-year survival rate above 70%. However, similar survival rates have been described in patients with tumors whose features fall outside of the Milan criteria, suggesting that it is a very restrictive model17. Other models that include factors not in the Milan criteria have incorporated biochemical markers, such as alpha-fetoprotein (AFP), to improve survival prediction and reduce the risk for post-LT recurrence18.
In the past, iCC was an absolute contraindication for LT, due to the high rate of tumor recurrence11,13 and the reported low survival rate (<25% at 5 years)19. In addition, diagnosis is often delayed, resulting in curative treatments no longer being an option20. In recent years, studies have shown that single iCC tumors ≤2cm result in a survival rate similar to HCC tumors that fit the Milan criteria20–21. The aim of our study was to evaluate the characteristics of the primary liver tumors and identify possible LT candidates at two centers belonging to the Hospital Universitario de la Universidad Autónoma de Nuevo León (UANL).
Materials and methodsStudy design and inclusion criteriaA retrospective observational study was conducted at the Hepatology Center (HC) and the University Center Against Cancer (UCAC), both of which belong to the Hospital Universitario “Dr. José Eleuterio González”, on patients with confirmed primary liver cancer, within the time frame of January 1, 2012, and December 31, 2018. The study included patients above 18 years of age, with or without cirrhosis, that had more than one medical follow-up consultation.
Study variablesThe demographic characteristics of the population, comorbidities, cause of cirrhosis, liver function classifications (Child–Pugh class in cirrhotic patients, model for end-stage liver disease [MELD] score), number of tumors, largest tumor diameter, and serum AFP level at diagnosis of HCC were evaluated. Our focus was exclusively on the primary liver tumors of HCC and iCC. We did not include cases of eCC. The Okuda22 and the Barcelona Clinic Liver Cancer (BCLC)23 classifications were employed to stage the HCC patients as follows: A-early, B-intermediate, C-advanced, and D-terminal. The Milan criteria16 and the University of California San Francisco (UCSF) criteria17 were applied to determine which patients were suitable for receiving a LT, and the AFP model17 was utilized to place the population into groups at low risk (≤2 points) and high risk (>2 points) for HCC recurrence post-LT.
The patients with iCC were subdivided into early (single tumor ≤2cm) and late (>2cm or multinodular±microvascular invasion and/or distant nodules20. The treatments received were listed and the patients that were evaluated as candidates for receiving a LT, and those that received LT, were registered. The survival rate of each group was documented.
Statistical analysisFor the statistical analysis, measures of central tendency (mean, standard deviation, percentages) were used. The Fisher’s exact test and the chi-square test were applied to the categorical variables and the Student’s t test and the Mann–Whitney U test were applied to the continuous variables. The Kaplan–Meier survival curves were compared using the log-rank test. Statistical significance was set at a p<0.05.
Ethical considerationsThe study was approved by the Research and Ethics Committee of the Hospital Universitario “Dr. José Eleuterio González” (register: HI18-00001).
Protection of human and animal subjects. The authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of data. The authors declare that they have treated all patient data with confidentiality and anonymity, following the protocols of their work center.
Right to privacy and informed consent. The authors declare that informed consent was not requested for the publication of this article because it contains no personal data that could identify the patients.
Study populationThere was a combined total of 189 patients from the two centers. Forty-two of the patients were excluded due to confirmation of another diagnosis and 38 were excluded due to lack of follow-up, leaving a total of 109 patients included in the study. The diagnosis of HCC and/or CC was confirmed through contrast-enhanced imaging studies (computed tomography or magnetic resonance imaging) and elevated AFP (HCC) or histopathology.
Of the entire population analyzed, 61 (56%) patients were seen at the HC and 48 (44%) at the UCAC. Mean patient age at diagnosis was 64±11.18 years (29–91) and 71 (65%) of the patients were men. At the HC, cirrhotic patients are screened every 6 months through ultrasound and AFP measurement. At the UCAC, the patients are not screened because they already have their cancer diagnosis, given that the center receives an open population for any type of previously identified neoplasm.
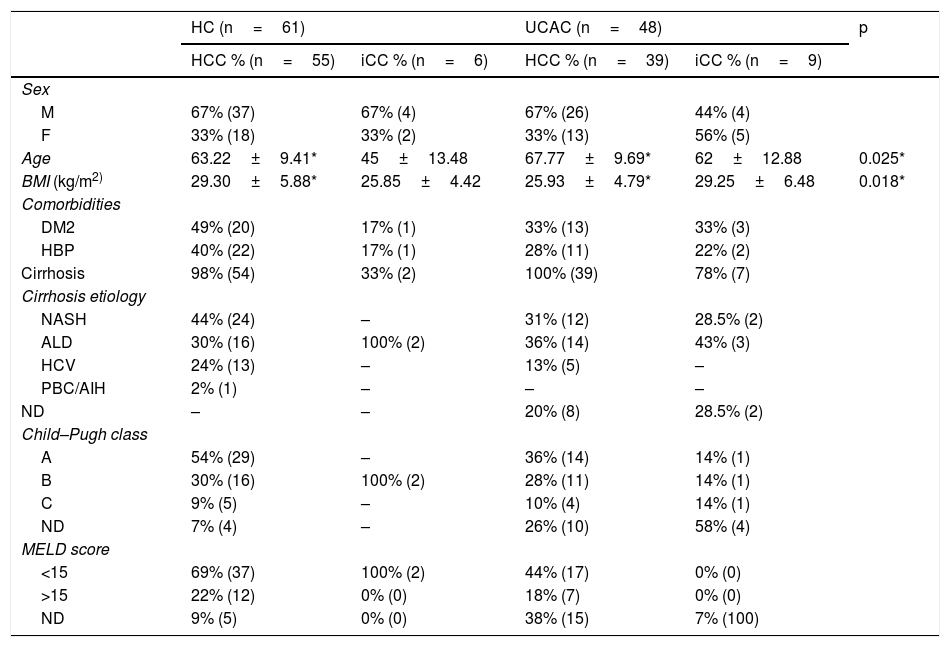
ResultsOf the combined population from the two centers, 94 (86%) patients were diagnosed with HCC. Sixty-seven percent (n=63) of the patients were men and the mean patient age was 65±9.69 years. Ninety-nine percent (n=93) of the patients with HCC had cirrhosis at diagnosis and NASH was the most frequent cause. Fifteen (14%) patients presented with iCC. Fifty-three percent were men, and the mean patient age was 56±14.89 years. Sixty percent (n=9) of the population with CC had cirrhosis and the most prevalent cause was ALD (Table 1). At the HC, 90% (n=55) of the patients were diagnosed with HCC and 10% (n=6) with iCC. In contrast, 81% (n=39) of the patients at the UCAC were diagnosed with HCC and 19% (n=9) with iCC. Thirty-four percent (n=37) of all the patients presented with DM2 and 33% (n=36) had high blood pressure (HBP). At the UCAC, 22% (n=2) of the cases of iCC had a history of cholecystolithiasis and cholecystectomy. The Child–Pugh class was determined at diagnosis in 85% (n=79) of the cirrhotic patients with HCC and 55% (n=5) of the patients with CC. Child–Pugh class A predominated in the patients with HCC. The majority of the patients from the two centers had a MELD score <15 (Table 1).
Demographics, etiologies, and functional classifications.
| HC (n=61) | UCAC (n=48) | p | |||
|---|---|---|---|---|---|
| HCC % (n=55) | iCC % (n=6) | HCC % (n=39) | iCC % (n=9) | ||
| Sex | |||||
| M | 67% (37) | 67% (4) | 67% (26) | 44% (4) | |
| F | 33% (18) | 33% (2) | 33% (13) | 56% (5) | |
| Age | 63.22±9.41* | 45±13.48 | 67.77±9.69* | 62±12.88 | 0.025* |
| BMI (kg/m2) | 29.30±5.88* | 25.85±4.42 | 25.93±4.79* | 29.25±6.48 | 0.018* |
| Comorbidities | |||||
| DM2 | 49% (20) | 17% (1) | 33% (13) | 33% (3) | |
| HBP | 40% (22) | 17% (1) | 28% (11) | 22% (2) | |
| Cirrhosis | 98% (54) | 33% (2) | 100% (39) | 78% (7) | |
| Cirrhosis etiology | |||||
| NASH | 44% (24) | – | 31% (12) | 28.5% (2) | |
| ALD | 30% (16) | 100% (2) | 36% (14) | 43% (3) | |
| HCV | 24% (13) | – | 13% (5) | – | |
| PBC/AIH | 2% (1) | – | – | – | |
| ND | – | – | 20% (8) | 28.5% (2) | |
| Child–Pugh class | |||||
| A | 54% (29) | – | 36% (14) | 14% (1) | |
| B | 30% (16) | 100% (2) | 28% (11) | 14% (1) | |
| C | 9% (5) | – | 10% (4) | 14% (1) | |
| ND | 7% (4) | – | 26% (10) | 58% (4) | |
| MELD score | |||||
| <15 | 69% (37) | 100% (2) | 44% (17) | 0% (0) | |
| >15 | 22% (12) | 0% (0) | 18% (7) | 0% (0) | |
| ND | 9% (5) | 0% (0) | 38% (15) | 7% (100) | |
ALD: alcoholic liver disease; BMI: body mass index; DM2: type 2 diabetes mellitus; NASH: nonalcoholic steatohepatitis; HBP: high blood pressure; HC: Hepatology Center; HCC: hepatocellular carcinoma; HCV: hepatitis C virus; iCC: intrahepatic cholangiocarcinoma; MELD: model for end-stage liver disease; ND: not determined; PBC/AIH: primary biliary cholangitis/autoimmune hepatitis; UCAC: University Center Against Cancer.
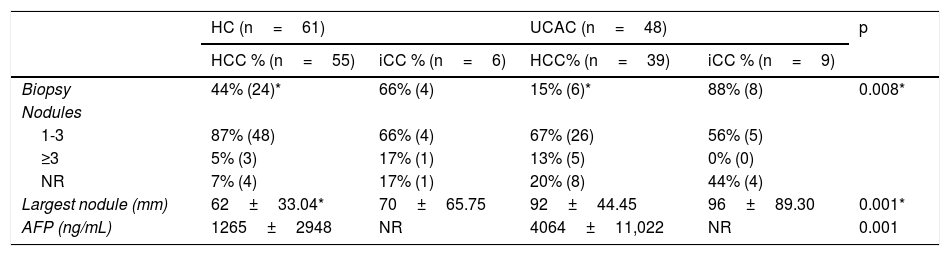
From both centers, the majority of the population analyzed (76%) had 1–3 tumor nodules (Table 2). A significant difference was found regarding the largest tumor diameter of HCC between the HC and UCAC patients. In 12/15 (80%) HC patients with iCC, diagnosis was made through biopsy, and an experienced radiologist interpreted a contrast-enhanced computed tomography scan with data suggestive of iCC in 3 patients. In 2 UCAC patients with iCC, the diagnosis was made, after a finding resulting from a cholecystectomy.
Tumor characteristics.
| HC (n=61) | UCAC (n=48) | p | |||
|---|---|---|---|---|---|
| HCC % (n=55) | iCC % (n=6) | HCC% (n=39) | iCC % (n=9) | ||
| Biopsy | 44% (24)* | 66% (4) | 15% (6)* | 88% (8) | 0.008* |
| Nodules | |||||
| 1-3 | 87% (48) | 66% (4) | 67% (26) | 56% (5) | |
| ≥3 | 5% (3) | 17% (1) | 13% (5) | 0% (0) | |
| NR | 7% (4) | 17% (1) | 20% (8) | 44% (4) | |
| Largest nodule (mm) | 62±33.04* | 70±65.75 | 92±44.45 | 96±89.30 | 0.001* |
| AFP (ng/mL) | 1265±2948 | NR | 4064±11,022 | NR | 0.001 |
AFP: alpha-fetoprotein; HC: Hepatology Center; HCC: hepatocellular carcinoma; iCC: intrahepatic cholangiocarcinoma; NR: not reported; UCAC: University Center Against Cancer.
AFP was determined in 74 patients from the two centers. Of those patients, 38 (51.3%) had levels below 100ng/mL, 16 (21%) had levels between 101 and 999ng/mL, and 20 (27%) had levels equal to or greater than 1000ng/mL. In the HC patients, the range was 1.07–12,378ng/mL and 0.79–52,477ng/mL in the UCAC patients (Table 2). With respect to CA 19-9 determination in the patients with iCC, results from 8/15 (53%) patients from the two centers showed a mean 85.11IU/mL (2–323IU/mL) in the HC patients and 952.2IU/mL (808.8–1000IU/mL) in the UCAC patients.
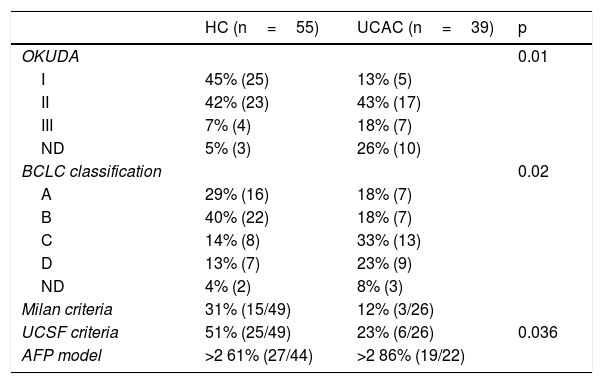
According to the HCC staging classifications, the Okuda I-II and BCLC A-B classifications predominated in the HC patients, whereas the Okuda II-III and BCLC C-D predominated in the UCAC patients (Table 3). In accordance with the Milan criteria and the UCSF criteria, between 33 and 51% of the HC patients could be considered candidates for orthotopic LT. In contrast, only 12–23% of the UCAC population met the criteria. Just 30% of the cases of HCC had an AFP model score ≤2 (Table 3). Between the two centers, 17/19 patients that fit the Milan criteria and 20/31 that fit the UCSF criteria had an AFP score ≤2. No patient outside of the Milan and the UCSF criteria had a low-risk AFP model score. Of the entire population, only one patient had early iCC characteristics (UCAC).
Staging and selection models for LT in patients with HCC.
| HC (n=55) | UCAC (n=39) | p | |
|---|---|---|---|
| OKUDA | 0.01 | ||
| I | 45% (25) | 13% (5) | |
| II | 42% (23) | 43% (17) | |
| III | 7% (4) | 18% (7) | |
| ND | 5% (3) | 26% (10) | |
| BCLC classification | 0.02 | ||
| A | 29% (16) | 18% (7) | |
| B | 40% (22) | 18% (7) | |
| C | 14% (8) | 33% (13) | |
| D | 13% (7) | 23% (9) | |
| ND | 4% (2) | 8% (3) | |
| Milan criteria | 31% (15/49) | 12% (3/26) | |
| UCSF criteria | 51% (25/49) | 23% (6/26) | 0.036 |
| AFP model | >2 61% (27/44) | >2 86% (19/22) |
AFP: alpha-fetoprotein; BCLC: Barcelona Clinic Liver Cancer; HC: Hepatology Center; HCC: hepatocellular carcinoma; LT: liver transplantation; UCAC: University Center Against Cancer; UCSF: University of California San Francisco.
Sixty-four percent (n=70) of the population analyzed received treatment. Thirty-six percent (n=39) of the patients did not undergo treatment within the study period for the following reasons: they were referred to another institution and/or city for treatment, they died before receiving treatment, they were still being evaluated to receive TACE or sorafenib at the study cutoff point, they refused treatment despite the insistence of their physician, or they could not afford the therapy. Thirty-two percent (n=10) of the HC patients with HCC received curative treatment (surgical resection, RFA, cryotherapy, LT) and 68% (n=21) received noncurative treatment (TACE, sorafenib). Of the HC patients that had iCC, only 3 received treatment (CT: capecitabin, gemcitabine, cisplatin). Eleven percent (n=3) of the UCAC patients with HCC received curative treatment (resection, cryoablation), 52% (n=14) received noncurative treatment (TACE, sorafenib), and 37% (n=10) received CT (folinic acid, fluorouracil, oxaliplatin±capecitabin, oxaliplatin). Of the patients with iCC that were treated, 89% (n=8) received CT (capecitabine, gemcitabine, cisplatin, fluorouracil) and 11% (n=1) died before receiving treatment.
During the study period, 15 (27%) patients, exclusively from the HC, were evaluated for LT and 6 of them underwent transplantation. Of the 9 remaining patients, one had tumor size progression and was outside of the Milan criteria; 2 unsuccessfully underwent TACE to lower their disease stage; one patient went to another center for LT; 4 finished the protocol and were on the waiting list; and one patient withdrew the statement of informed consent. With respect to the study of the liver explant, 100% (n=6) of the transplanted patients had one tumor, with a mean size of 30 (10–50) mm. All the patients met the Milan and the UCSF criteria and had an AFP score ≤2 points.
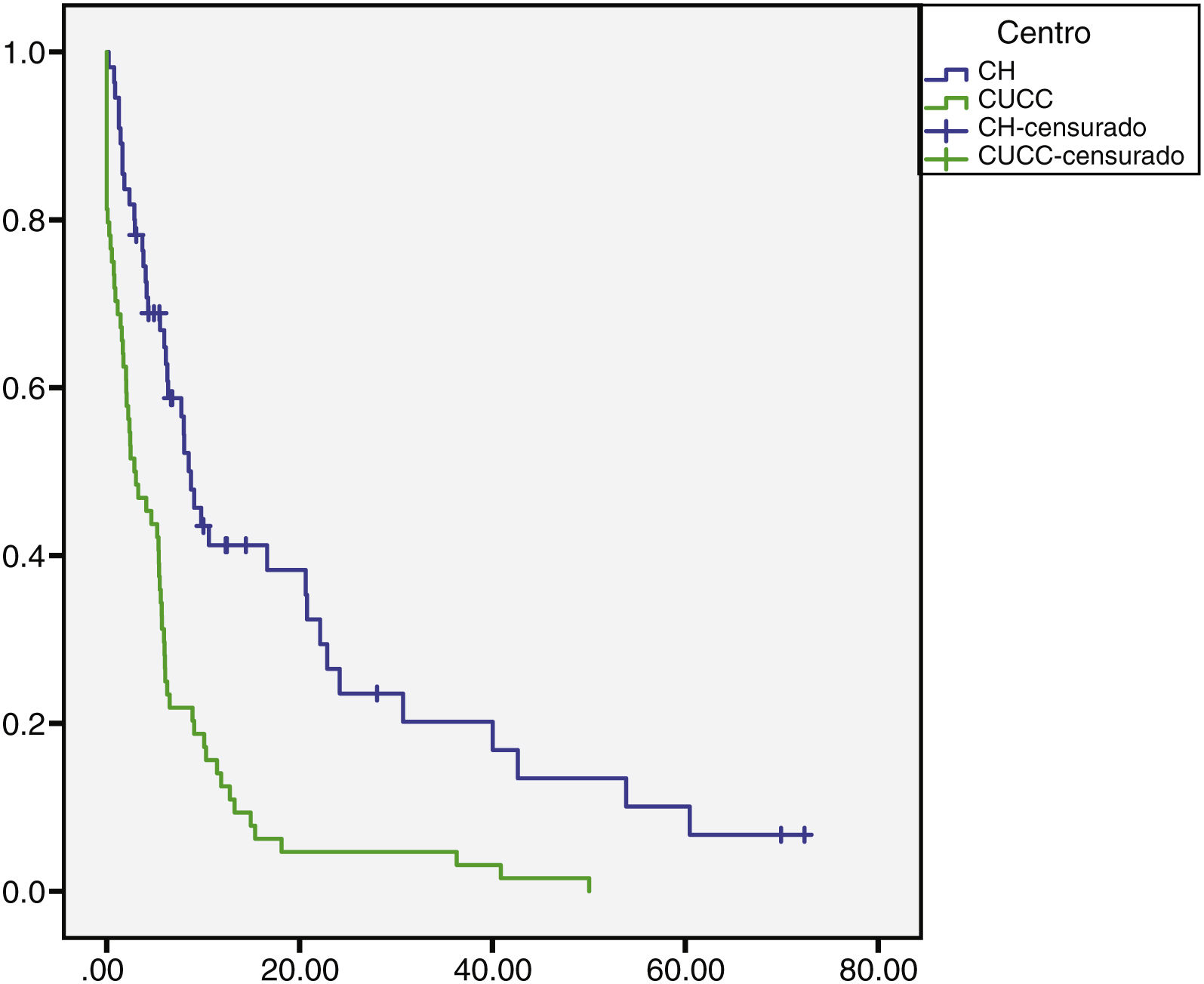
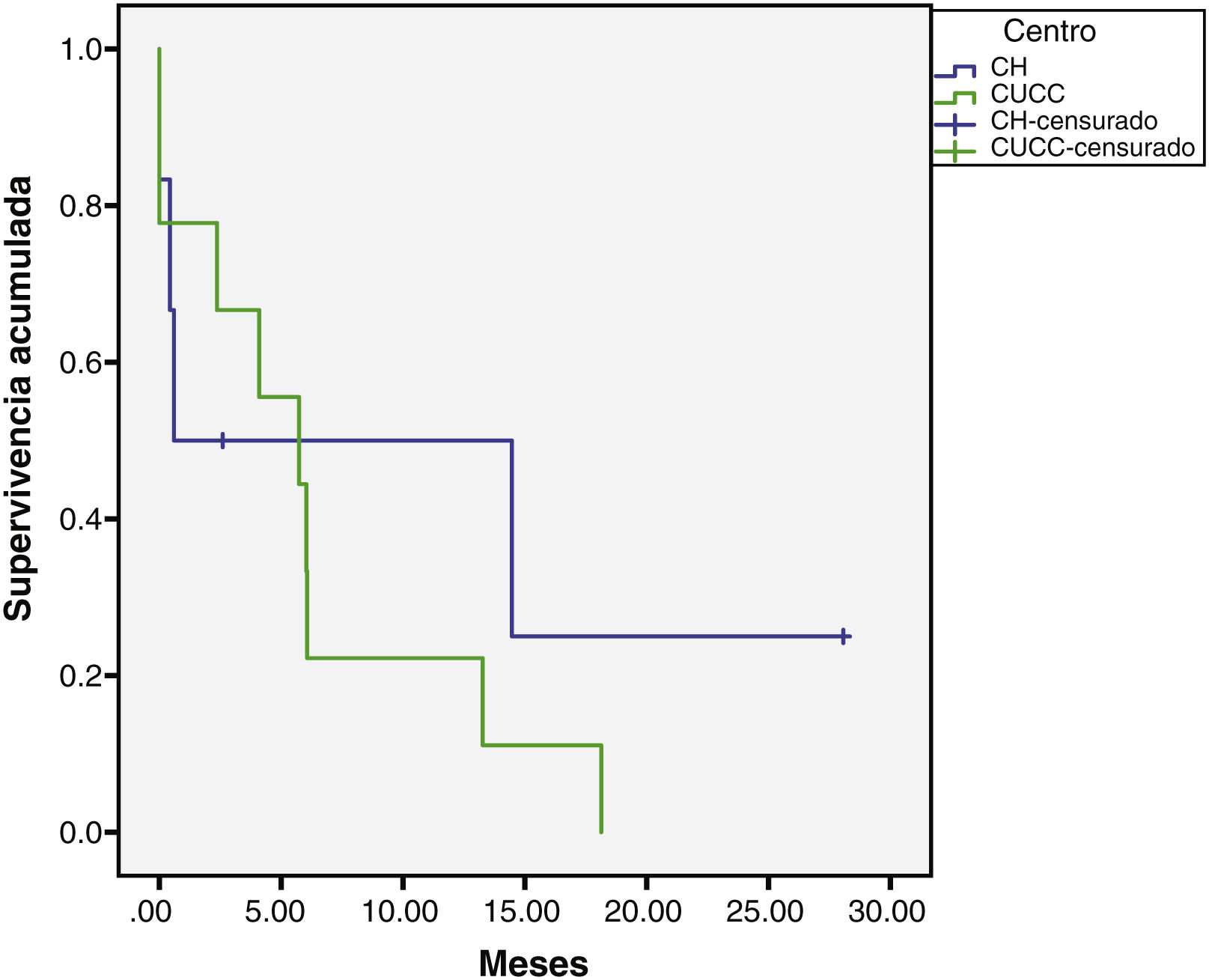
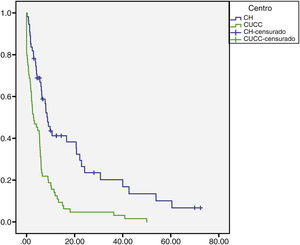
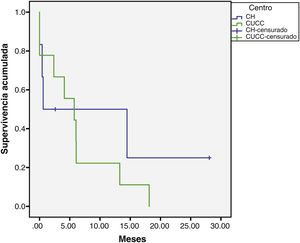
The median overall follow-up duration was 5.7 months (0.10–72). At the HC, follow-up was 6.8 months (0.76–72) in the patients with HCC and 1.6 months (0.26–28) in the patients with iCC, whereas at the UCAC, follow-up was 5.23 months (0.10–50) in the patients with HCC and 5.73 months (1.43–18.13) in the patients with iCC (Figs. 1 and 2). Thirty-four percent (n=21) of the HC patients were lost to follow-up after 6 years and 100% of the UCAC patients were lost to follow-up after 4 years. All the patients lost to follow-up had been reached out to by telephone, but with no success. Of the entire population, 14% (n=15) died during the study time frame, and 60% (n=9) of those deaths were due to hepatic causes.
Survival rate in months of the patients with HCC at the HC and UCAC.
Patients from the HC had a statistically significant higher survival rate than the patients from the UCAC (log-rank test, p=0.000).
HC: Hepatology Center; HCC: hepatocellular carcinoma; UCAC: University Center Against Cancer.
Of the 6 cases that underwent LT, two of the patients died. One of those patients presented with septic shock 2 weeks after transplant. The other had undergone extended hepatectomy due to HCC 2 years before the LT. Microvascular invasion was identified in the liver explant and 9 months after transplantation the disease recurred. The patient was treated with Nexavar and survived for 3 years and 5 months.
Discussion and conclusionsThe present retrospective study was conducted at the HC and UCAC, two centers belonging to the Hospital Universitario UANL. The HC is a national referral hepatology center for patients with liver disease and the UCAC receives an open oncologic population of patients newly diagnosed with liver cancer. A population of 109 patients with primary liver cancer were analyzed, 94 (86%) with HCC and 15 (14%) with iCC.
Cirrhosis is a risk factor that is frequently associated with the development of HCC5, which is not necessarily the case for CC. Tyson et al.7 reported a prevalence of cirrhosis below 10% in patients with CC, and in our study, 9/15 (60%) of the patients with CC had cirrhosis. Historically, the most frequent causes of cirrhosis have been HCV and ALD, but an important increase in the number of patients with NASH has recently been seen, given the current epidemic of obesity and DM2 in Mexico24 and Latin America15. The results of the present study concur with that new trend of NASH as the main cause of cirrhosis, in patients with HCC. Strikingly, in the study by Cisneros et al.25, the main causes of cirrhosis were HCV, HBV, and ALD between 2008 and 2014. In our study, conducted within the time frame of 2012 and 2018, the most frequent etiology was NASH, followed by ALD and HCV, and there was a statistically significant difference between centers in relation to the staging of the patients with HCC. In the study by Riaz et al.26 conducted at an oncology center, 41% of the population analyzed had BCLC stage C disease, similar to the staging we found at the UCAC. In another Mexican study that analyzed patients with HCC from the northeastern and central regions of the country25, the majority (71%) of patients had BCLC stages B, C, and D, and similarly, 70% of our patients had advanced B, C, and D stages of disease. Survival in stages A, B, and C was between 8.9 and 16.5 months in the Cisneros study25, and similarly in our study, it varied from 11.3 to 14.8 months. However, in stage D, our patients had a survival of 8.9 months, compared with 4.5 months in the study by Cisneros et al.25
Different kinds of patients are seen at the HC and UCAC. In the HC, there is a population with liver disease in follow-up that every 6 months undergoes Doppler ultrasound, precisely because the presence of cirrhosis is a risk factor in itself for the development of HCC and CC. In the UCAC, the patients arrive with more advanced tumor stages because it is a general oncology center and the patients are newly diagnosed. Both centers belong to the Hospital Universitario “Dr. José Eleuterio González”, UANL.
The screening that the HC patients with cirrhosis underwent impacted patient survival. The performance of imaging studies (the most accessible of which is ultrasound) every 6 months, with or without AFP measurement, is universally accepted3,5. AFP has long been used as an important post-treatment recurrence predictor in patients with HCC, even though it is elevated in only 50–60% of the patients27. It has been included in different selection models for LT. Duvoux et al.18 and Mazzaferro et al.28 added AFP to their models to improve survival and post-LT HCC recurrence. The majority of the HC patients analyzed had an AFP level that was considered low risk, unlike the UCAC patients.
Due to the advanced disease seen in the UCAC patients with HCC, the majority received noncurative treatment and none of them met the criteria for LT. At the HC, 15 patients with HCC began the evaluation process for LT, 6 of whom underwent transplantation and 4 of whom were placed on the waiting list, reflecting the difficulty in our environment for patients to have access to that therapy.
We identified 15 cases of iCC in 7 years in Northern Mexico and Chinchilla et al.8 found 18 cases of iCC over 11 years in Central Mexico, giving an idea of the prevalence of that disease in the two areas. The increase in liver diseases in recent years, such as HCV and fatty liver disease, could explain the increase in iCC. Strikingly, in their study, Chinchilla et al.8 found cirrhosis in only 1/18 (5.5 %) cases with iCC, whereas in our study, 30% of the cases at the HC and 78% of the cases at the UCAC had cirrhosis. We could not make generalizations, given the small sample size of our study. Five-year survival of iCC is below 5% and one-year survival is 75%29. According to epidemiologic data from the United States, the Hispanic population has a higher prevalence of iCC and better survival, compared with non-Hispanic white, non-Hispanic black, native American Indian, native Alaskan, and native Asian/Pacific populations29. According to records from 1973 to 2011 at 18 registries of the United States, iCC occurs in 28% of that country’s population29.
At the HC, male sex predominated in the patients with iCC and female sex was slightly predominant at the UCAC. Nevertheless, the number of patients was too small to draw conclusions. The majority of the patients with iCC from the two centers had the risk factor of cirrhosis. Interestingly, there were more cases of CC (18.7%) (9/48) in the patients at the UCAC than in the patients at the HC (9.8%) (6/61). Prevalence of CC reported in the literature ranges from 11 to 15% of malignant liver diseases30. Said prevalence at the UCAC is perhaps a better reflection of what occurs in an open population, because the HC is a national referral center and there could be more bias in the analysis of such a population. On the other hand, survival in the patients with iCC was similar to that reported in other national studies8, given that patients are diagnosed at more advanced disease stages. Chinchilla et al. reported survival of 286 days (9.5 months), whereas survival in our patients was 20 (UCAC) and 30 (HC) months.
The retrospective design of our study was a limitation. Follow-up loss occurred at both centers, limiting the knowledge of the outcome in several patients. Most likely those patients died, given their advanced stage of disease. Other limitations were the differences in criteria for treating the patients at the two centers, a situation that is currently being worked on, to establish broader communication for the purpose of unifying criteria.
In conclusion, the most frequent primary liver cancer at the two centers was HCC. With respect to iCC, more cases were detected at the UCAC than at the HC. The patients with HCC treated at the HC had earlier stages of disease and should be opportunely referred to specialized hepatology centers. A much higher number of patients seen at the HC met the Milan criteria and the UCSF criteria for LT and only HC patients underwent transplantation. Patients with a low-risk French AFP model score had a higher probability of survival than the patients with a high-risk score.
Financial disclosureNo financial support was received in relation to this study/article.
Conflict of interestThe authors declare that there is no conflict of interest.
Please cite this article as: Rojas-Pintor KP, Arizmendi-Villarreal MA, Aparicio-Salas JE, Moreno-Peña DP, Hernández-Barajas D, Cordero-Pérez P, et al. Diferencias de la presentación y tratamiento en las neoplasias primarias de hígado en un centro de hepatología y un centro oncológico. Revista de Gastroenterología de México. 2021;86:370–377.