Gastrointestinal angiodysplasia (GIAD) is the most common vascular anomaly in the gastrointestinal (GI) tract, yet little is known about the factors favoring their bleeding. Our study aim was to determine the characteristics of patients with GIAD lesions in a Tunisian population and identify the risk factors of bleeding.
Patients and methodsA retrospective study was carried out from January 2010 to February 2020 at a tertiary care medical center in Tunisia. Clinical and endoscopic data were collected from each patient’s medical reports. We divided the patients into two groups: group A, patients with symptomatic GIAD; and group B, patients with incidental lesions. Group A was subsequently divided into two subgroups, according to the presence or absence of recurrent bleeding. The groups were compared by clinical, laboratory, and endoscopic features.
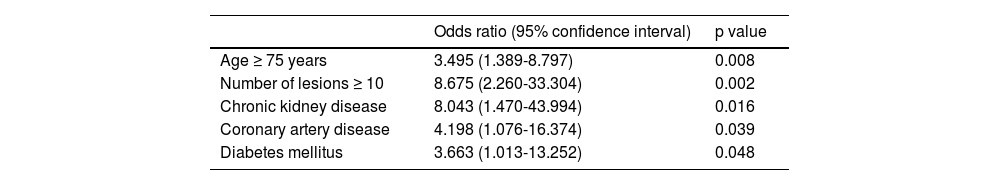
ResultsGIAD was diagnosed in 114 patients, with a mean age of 70 ± 13.3 years. GIAD lesions were mainly located in the colon (n = 72, 63%). Fifty-four patients (47%) presented with GIAD-related bleeding. The bleeding diagnosis was made during endoscopic procedures by visualizing active bleeding and the stigmata of recent hemorrhage in 10 (18.5%) and 12 (22.2%) cases, respectively. Most of the patients were treated by argon plasma coagulation (93%). Predictive factors of bleeding were age > 75 years, number of lesions >10, chronic kidney disease, diabetes mellitus, and coronary artery disease (p: 0.008; 0.002; 0.016; 0.048; and 0.039, respectively).
ConclusionKnowledge of the predictive factors of bleeding aids endoscopists in the decision-making process in cases of angiodysplasia.
La angiodisplasia gastrointestinal (ADGI) es la anomalía vascular más común en el tracto gastrointestinal (GI), sin embargo, poco se sabe de los factores que favorecen su sangrado. El objetivo de nuestro estudio fue determinar las características de los pacientes con lesiones de ADGI en una población tunecina e identificar los factores de riesgo de sangrado.
Pacientes y métodosSe realizó un estudio retrospectivo de enero del 2010 a febrero del 2020 en un centro médico de tercer nivel de Túnez. Los datos clínicos y endoscópicos fueron obtenidos de los reportes médicos de cada uno de los pacientes. Dividimos a los pacientes en dos grupos: el grupo A fue conformado con pacientes con ADGI sintomática y el grupo B con pacientes con lesiones incidentales. El grupo A fue dividido a su vez en dos subgrupos, según la presencia o ausencia de sangrado recurrente. Los grupos fueron comparados por características clínicas, de laboratorio y endoscópicas.
ResultadosSe diagnosticó ADGI en 114 pacientes, con una edad promedio de 70 ± 13.3 años. Las lesiones de ADGI se localizaron principalmente en el colon (n = 72, 63%). Cincuenta y cuatro pacientes (47%) presentaron sangrado asociado a ADGI. El diagnóstico de sangrado se realizó durante los procedimientos endoscópicos al visualizar sangrado activo y estigmas de hemorragia reciente en 10 (18.5%) y 12 (22%.2%) casos, respectivamente. La mayoría de los pacientes fueron tratados con coagulación con plasma de argón (93%). Los factores predictores de sangrado fueron la edad > 75 años, el número de lesiones > 10, enfermedad renal crónica, diabetes mellitus y enfermedad de las arterias coronarias (p: 0.008; 0.002; 0.016; 0.048; y 0.039, respectivamente).
ConclusiónConocer los factores de riesgo de sangrado ayuda a los endoscopistas en el proceso de toma de decisiones en casos de angiodisplasia.
Gastrointestinal angiodysplasia (GIAD) is the most common vascular abnormality in the gastrointestinal (GI) tract, defined as pathologically dilated communications between veins and capillaries.1 Histologically, it consists of an accumulation of thin-walled veins, venules, and capillaries along the endothelium in the mucosa and submucosa. Those vessels lack a smooth muscle layer, leaving them easily prone to bleeding.1
Endoscopically, GIAD lesions are flat or slightly raised, bright red in color, and well circumscribed or fernlike stellar lesions measuring 2 to 10 mm in diameter.1 They can occur at multiple sites within the GI tract, including the colon and the stomach, where they can be easily diagnosed. It is the small bowel angiodysplasia lesions that remain a diagnostic and therapeutic challenge, in spite of the technologic advances in the field of small bowel exploration made over the past 2 decades. The distribution of GIAD lesions in the GI tract is not well known, as most studies do not include small bowel endoscopic assessment.2 GIAD lesions account for up to 5% of all causes of GI bleeding. In patients with obscure GI bleeding, which is defined by bleeding from a source that cannot be identified through upper or lower endoscopy, small bowel angiodysplasia is found in approximately 40% of examinations.3 In addition to increasing age, morbidities, such as aortic stenosis, renal failure, cirrhosis, von Willebrand disease, liver cirrhosis, and pulmonary disease, have been reported to be predisposing factors for GIAD.4 Little is currently known about the risk factors for GIAD lesion bleeding. However, the identification of those factors can aid in predicting and preventing both the occurrence and recurrence of GIAD-related bleeding. In fact, patients with a high-risk profile would benefit from preemptive endoscopic treatment, close monitoring, and possibly from early prescription of prophylactic medical therapy. Therefore, the aim of our study was to describe the clinical features of patients with GIAD lesions in a Tunisian population and to determine the risk factors promoting GIAD lesion bleeding.
MethodsPatientsThe study was designed as a single-center chart review. The reports of all upper, lower, and small bowel endoscopic procedures performed within the time frame of January 2010 to February 2020 at the Endoscopy Unit of the Taher Maamouri University Hospital (Nabeul, Tunisia) were reviewed. We included patients with identified GIAD, with or without signs of bleeding. We did not include patients that had other vascular malformations, such as antral vascular ectasia, patients that had incomplete endoscopic procedures, or patients with poor bowel preparation, nor did we include patients with GI bleeding or anemia that could be explained by other lesions (such as variceal bleeding, peptic ulcers, or esophagitis), unless there was active bleeding or stigmata of hemorrhage around the GIAD lesion, during the endoscopy.
Data collectionWe collected the clinical data from each patient’s medical records and endoscopy reports. In cases of incomplete records or charts, the patients or their relatives were contacted, to obtain the appropriate variables. The following were the main characteristics collected for each patient: age, sex, smoking status, alcohol consumption (in grams per day), medical history (arterial hypertension, coronary artery disease, valvular heart disease, aortic stenosis, atrial fibrillation, type 2 diabetes mellitus, dyslipidemia, chronic kidney disease [CKD] defined by a glomerular filtration rate below 60 ml/min/1.73 m², and end-stage renal disease) and medication history (non-steroidal anti-inflammatory drugs [NSAIDs], platelet anti-aggregant therapy, and anti-coagulants). We also determined the indication for the endoscopic procedure and the need for hospital admission due to GI bleeding. In addition, we determined the following biological features, in patients with anemia or GI bleeding: creatinine level, platelet count, prothrombin time, and hemoglobin level. Anemia was defined by a hemoglobin level of less than 13 g/dl for men and 12 g/dl for women.
The following endoscopic features were determined in all patients: location, number, and size of the GIAD lesions. In the cases of multiple GIAD lesions, the size of the largest lesion for each segment of the GI tract was used. We also determined if there was active bleeding or stigmata of recent hemorrhage around the GIAD lesions, defined by adherent clots or pigmented spots. All treatments our patients received were determined (medical, endoscopic, and surgical). Conservative management consisted of blood transfusions, if needed; discontinuation of antiplatelets, anticoagulants, or NSAIDs; and the correction of hemostatic abnormalities. Medical treatment consisted of octreotide prescription. Octreotide dose and administration route, as well as side effects, were determined.
The endoscopic management of GIAD lesions at our unit is based on argon plasma coagulation (APC). All APC sessions were performed with a standard gastroscope, colonoscope, and duodenoscope (Olympus Corporation, Tokyo, Japan) with a working channel of at least 2.8 mm. APC was applied until all visible GIAD lesions were completely coagulated. The number of APC sessions and the time interval between them were determined, as well as any side effects or complications.
Design and definitionsThe patients were divided into 2 groups: group A and group B. Group A consisted of patients with symptomatic GIAD lesions. The diagnosis of bleeding from GIAD lesions was made, if active bleeding or stigmata of hemorrhage were visualized during endoscopy or if the patient presented with GI bleeding or anemia and no other potential causes of GI bleeding were found. Group B consisted of patients with asymptomatic GIAD lesions (patients with no bleeding or anemia). To determine the risk factors for GIAD bleeding, we compared the clinical, endoscopic, and laboratory features of the group A and group B patients.
Statistical analysisThe continuous variables were expressed as mean ± standard deviation, for those with normal distribution, and as median and interquartile range for those that failed the normality test. The Kolmogorov-Smirnov test was used to verify continuous variable normality. The qualitative variables were expressed as numbers and percentages and compared by the chi-square test or Fisher’s exact test. When developing the final model, we investigated all possible combinations of the candidate variables whose p values were < 0.2 in the univariate analysis. Two-tailed statistical tests were applied, and a p value < 0.05 was considered statistically significant. The analysis was performed, using the SPSS version 22 statistical software program for Windows.
Ethical considerationsInformed consent was not requested for the publication of this article, because the study was retrospective and no personal data that could identify the patients was published. The study was approved by the ethics committee of the Monastir Faculty of Medicine. The principles of the Declaration of Helsinki were respected throughout the study.
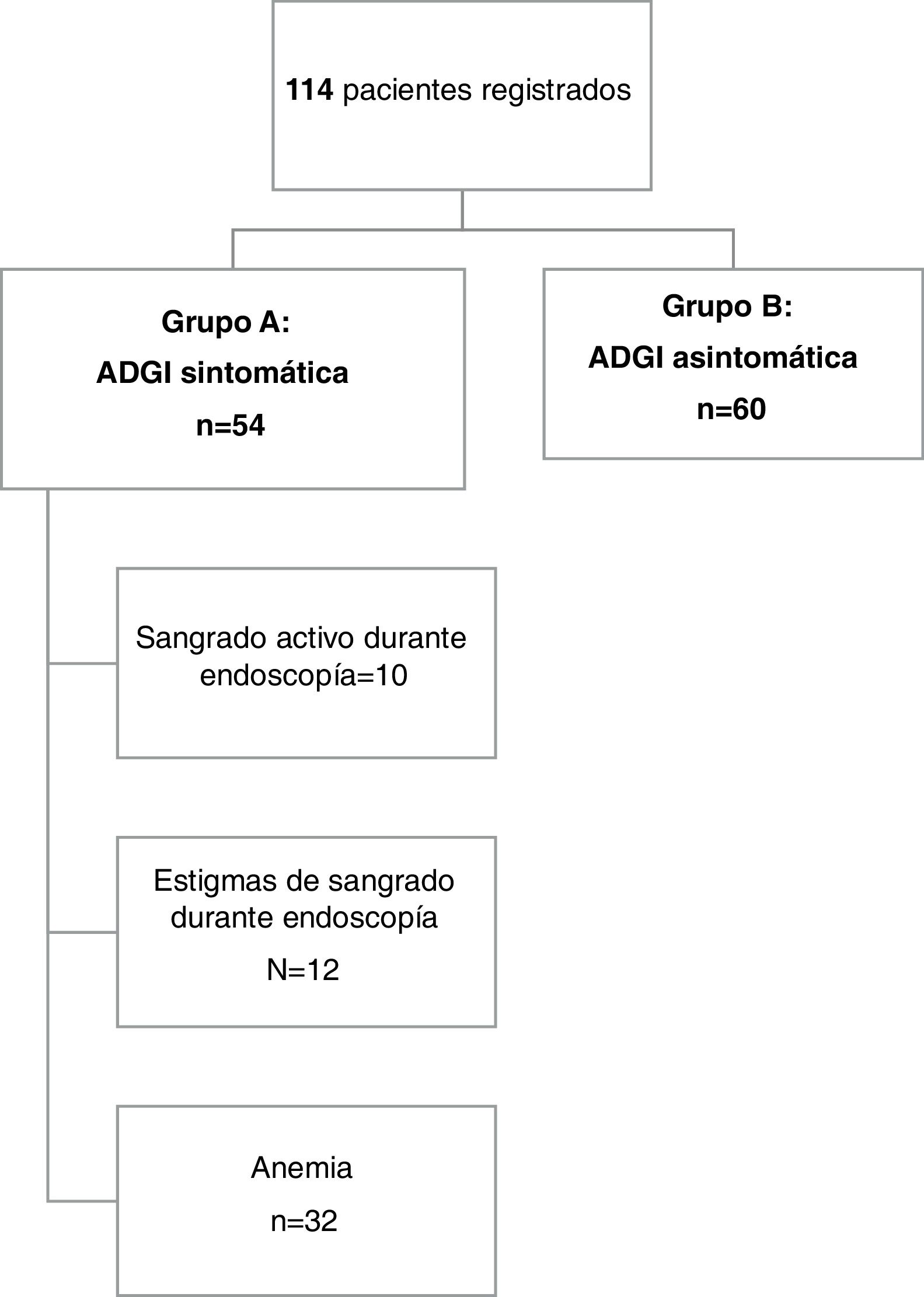
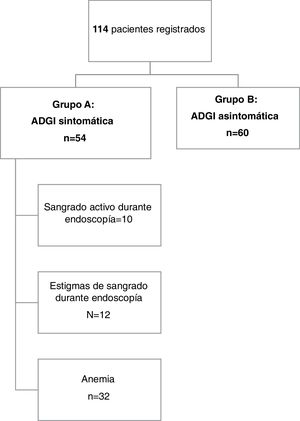
ResultsDescriptive studyFig. 1 provides an overview of the patients enrolled in the study. A total of 114 patients were included in the analysis. The mean age of the patients was 70.1 years ± 13.3, with a range of 25 to 95 years. Forty-one percent of the patients (n = 47) were 75 years of age or older and 62.3% of them were men (71 patients). Chronic alcohol consumption was reported by 5 patients (4.4%) and 19.3% of our population were smokers.
Seventy-eight patients (68.4%) had pre-existing medical conditions and only 36 patients had one chronic disease. Seventeen patients (14.9%) had CKD, 10 of whom (8.8%) were undergoing hemodialysis. Cardiovascular diseases were noted in 78 patients (68.4%). Coronary artery disease, arterial hypertension, and aortic stenosis were found in 19 (16.7%), 47 (41.3%), and 3 (2.6%) patients, respectively. None of the patients with aortic stenosis had valve replacement. Cirrhosis was found in 12 patients and was related to hepatitis B in 9 patients (8%) and hepatitis C in 3 cases (2.6%). Seven patients (6.1%) had chronic obstructive pulmonary disease. One patient was followed since childhood for von Willebrand disease. Eighteen patients (15.8%) were taking drugs that could interfere with primary or secondary hemostasis. Nine patients (7.9%) were taking acenocoumarol. In all nine cases, the international normalized ratio values were within the therapeutic range.
Overall, symptomatic GIAD lesions (group A) were identified in 54 patients (47.4%). Twenty-three patients (42.6%) presented with anemia and the remaining 31 patients (57.4%) presented with overt bleeding. Melena was the predominant form of GI bleeding in group A (n = 25, 46.3%), followed by hematemesis (n = 4, 7.4%) and hematochezia (n = 2, 3.7%).
The diagnosis of bleeding was made during endoscopic procedures by visualizing active bleeding or stigmata of recent hemorrhage in 10 (18.5%) and 12 (22.2%) cases, respectively.
In the 32 remaining cases, patients presented with hypochromic microcytic anemia or overt bleeding and no other lesions other than GIAD in both the upper and lower GI tract that could cause GI bleeding. The mean hemoglobin level in group A was 6.4 g/dl (± 2.3), ranging from 1.8 to 15 g/dl. Six patients had a prothrombin time value of less than 50%, 2 of whom were cirrhotic. The remaining 4 patients were on acenocoumarol, but no over-anticoagulation was documented.
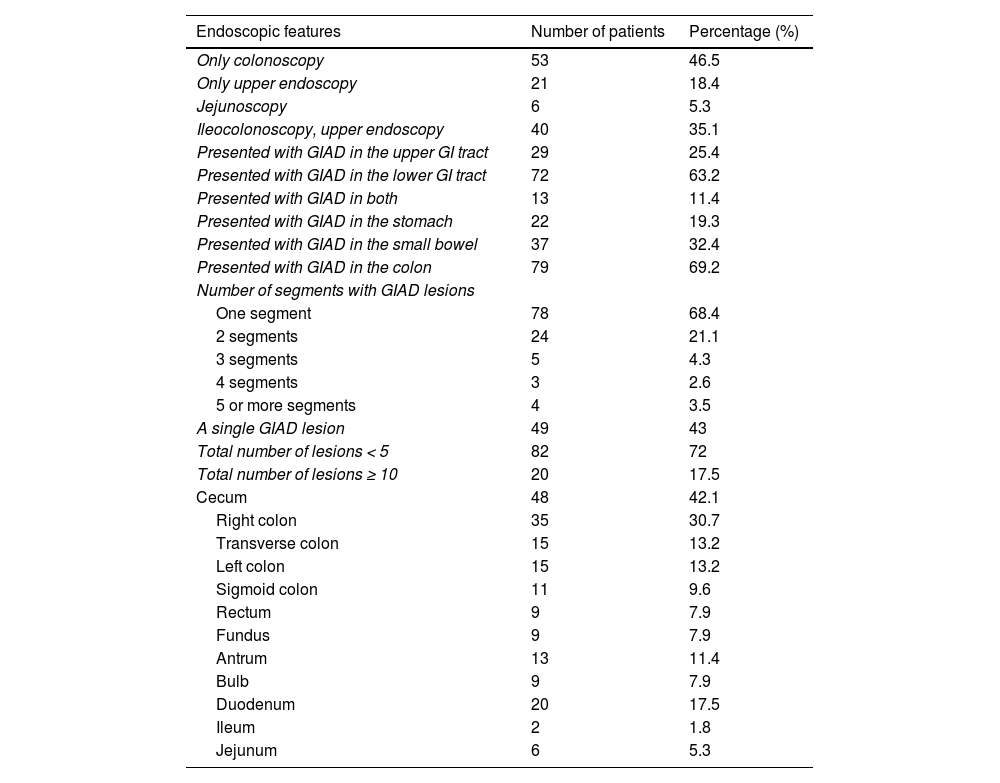
Regarding the endoscopic procedures, 53 patients (46.5%) solely underwent colonoscopy and 21 patients (18.4%) solely underwent upper GI endoscopy. In all the colonoscopy reports included in the study, the cecum was explored, and bowel preparation was judged as good or excellent by the endoscopist. Jejunoscopy was performed on 6 patients that had obscure bleeding. The distribution of the GIAD lesions involved the following sites: the stomach (fundus and antrum) in 22 cases (19.3%), the small bowel (bulb, duodenum, jejunum, and ileum) in 37 cases (32.4%), and the colon in 79 cases (69.2%). The following GI segments were involved: fundus, antrum, bulb, duodenum, jejunum, ileum, colon, and rectum. Endoscopic findings are depicted in Table 1. With respect to lesion number, GIAD was unique in 49 patients (43%) and there were 10 or more lesions in 20 cases (17.5%). Lesion size was estimated in 98 endoscopy reports and ranged from 1 to 20 mm.
Endoscopic features of patients with GIAD lesions.
| Endoscopic features | Number of patients | Percentage (%) |
|---|---|---|
| Only colonoscopy | 53 | 46.5 |
| Only upper endoscopy | 21 | 18.4 |
| Jejunoscopy | 6 | 5.3 |
| Ileocolonoscopy, upper endoscopy | 40 | 35.1 |
| Presented with GIAD in the upper GI tract | 29 | 25.4 |
| Presented with GIAD in the lower GI tract | 72 | 63.2 |
| Presented with GIAD in both | 13 | 11.4 |
| Presented with GIAD in the stomach | 22 | 19.3 |
| Presented with GIAD in the small bowel | 37 | 32.4 |
| Presented with GIAD in the colon | 79 | 69.2 |
| Number of segments with GIAD lesions | ||
| One segment | 78 | 68.4 |
| 2 segments | 24 | 21.1 |
| 3 segments | 5 | 4.3 |
| 4 segments | 3 | 2.6 |
| 5 or more segments | 4 | 3.5 |
| A single GIAD lesion | 49 | 43 |
| Total number of lesions < 5 | 82 | 72 |
| Total number of lesions ≥ 10 | 20 | 17.5 |
| Cecum | 48 | 42.1 |
| Right colon | 35 | 30.7 |
| Transverse colon | 15 | 13.2 |
| Left colon | 15 | 13.2 |
| Sigmoid colon | 11 | 9.6 |
| Rectum | 9 | 7.9 |
| Fundus | 9 | 7.9 |
| Antrum | 13 | 11.4 |
| Bulb | 9 | 7.9 |
| Duodenum | 20 | 17.5 |
| Ileum | 2 | 1.8 |
| Jejunum | 6 | 5.3 |
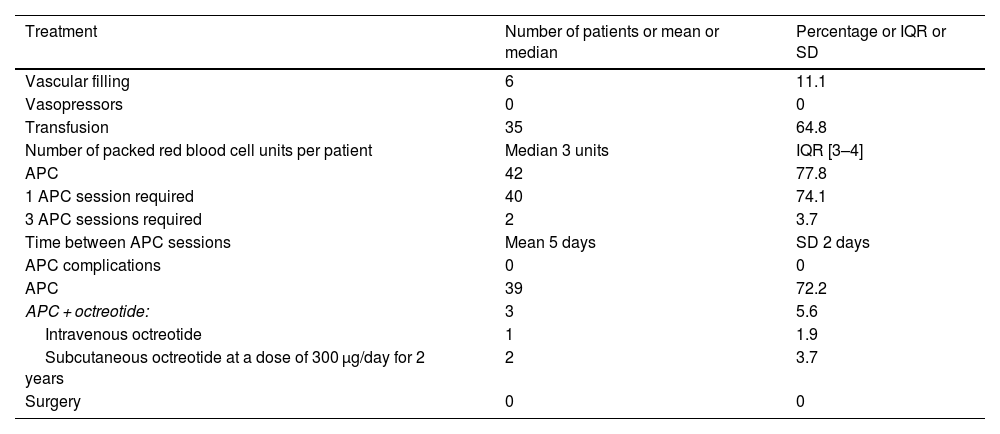
Of the 54 group A patients, 46 (85.2%) required hospital admission, 6 (11%) of whom had initial hemodynamic instability. In all six cases, the use of vasopressor drugs was not needed because the patients rapidly responded to vascular filling. Of the 54 patients, 35 (64.8%) required blood transfusion.
Table 2 depicts group A patient management. Of the 60 patients whose GIAD lesions were incidentally found, 14 were treated with APC during the same procedure. No other sessions were planned.
Treatments received by patients presenting with GIAD-related bleeding.
| Treatment | Number of patients or mean or median | Percentage or IQR or SD |
|---|---|---|
| Vascular filling | 6 | 11.1 |
| Vasopressors | 0 | 0 |
| Transfusion | 35 | 64.8 |
| Number of packed red blood cell units per patient | Median 3 units | IQR [3–4] |
| APC | 42 | 77.8 |
| 1 APC session required | 40 | 74.1 |
| 3 APC sessions required | 2 | 3.7 |
| Time between APC sessions | Mean 5 days | SD 2 days |
| APC complications | 0 | 0 |
| APC | 39 | 72.2 |
| APC + octreotide: | 3 | 5.6 |
| Intravenous octreotide | 1 | 1.9 |
| Subcutaneous octreotide at a dose of 300 µg/day for 2 years | 2 | 3.7 |
| Surgery | 0 | 0 |
APC: argon plasma coagulation; IQR: interquartile range; SD: standard deviation.
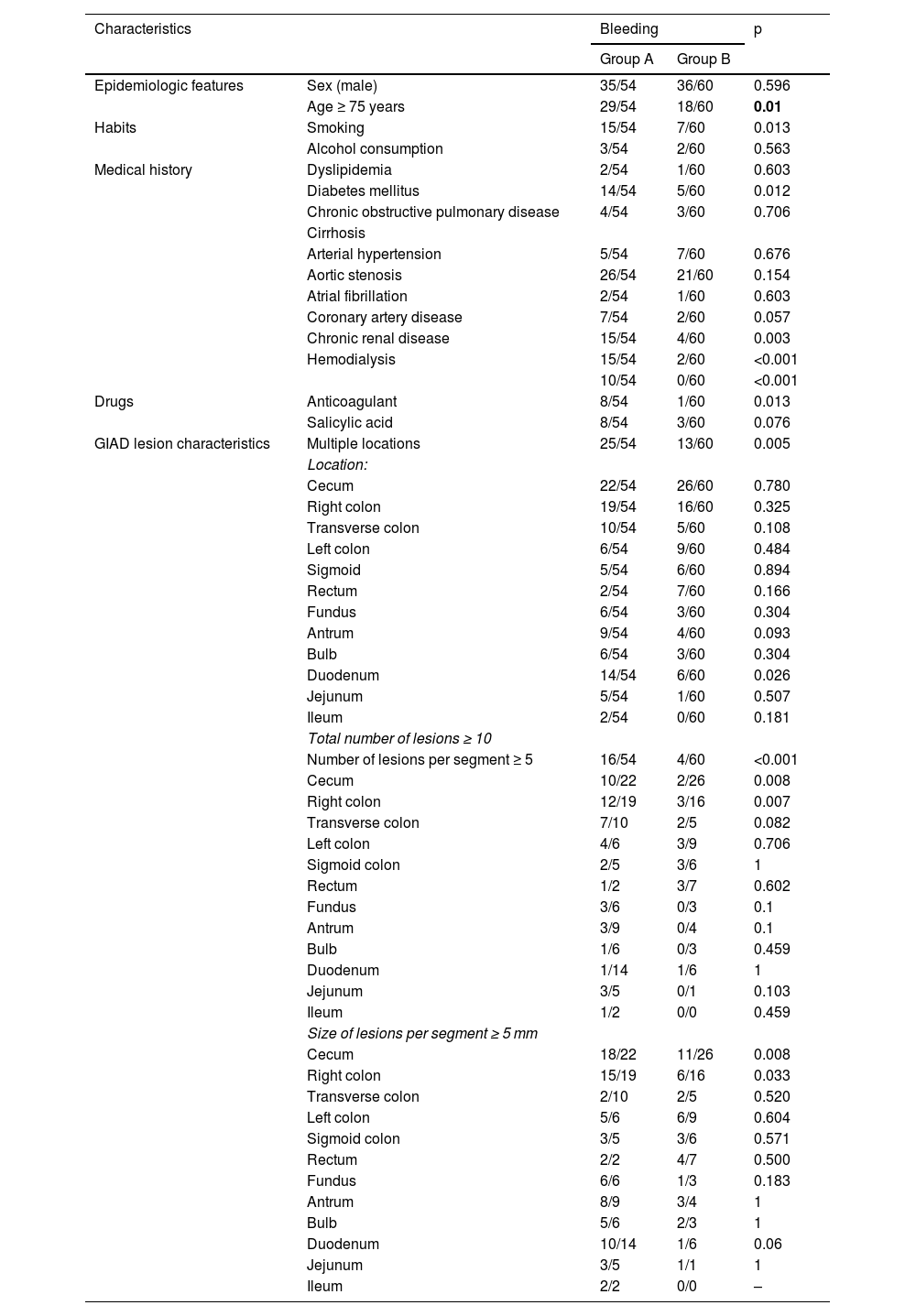
Table 3 summarizes the univariate study of the potential risk factors for GIAD bleeding. Group A and group B patients had the same sex distribution (p = 0.596). There was no significant difference in the distribution of GIAD lesions throughout the GI tract, except in the duodenum. In the multivariate analysis, the independent risk factors for GIAD-related bleeding were age ≥ 75 years, a total of ≥ 10 lesions, CKD, diabetes mellitus, and coronary artery disease (Table 4).
Risk factors for GIAD bleeding: univariate study.
| Characteristics | Bleeding | p | ||
|---|---|---|---|---|
| Group A | Group B | |||
| Epidemiologic features | Sex (male) | 35/54 | 36/60 | 0.596 |
| Age ≥ 75 years | 29/54 | 18/60 | 0.01 | |
| Habits | Smoking | 15/54 | 7/60 | 0.013 |
| Alcohol consumption | 3/54 | 2/60 | 0.563 | |
| Medical history | Dyslipidemia | 2/54 | 1/60 | 0.603 |
| Diabetes mellitus | 14/54 | 5/60 | 0.012 | |
| Chronic obstructive pulmonary disease | 4/54 | 3/60 | 0.706 | |
| Cirrhosis | ||||
| Arterial hypertension | 5/54 | 7/60 | 0.676 | |
| Aortic stenosis | 26/54 | 21/60 | 0.154 | |
| Atrial fibrillation | 2/54 | 1/60 | 0.603 | |
| Coronary artery disease | 7/54 | 2/60 | 0.057 | |
| Chronic renal disease | 15/54 | 4/60 | 0.003 | |
| Hemodialysis | 15/54 | 2/60 | <0.001 | |
| 10/54 | 0/60 | <0.001 | ||
| Drugs | Anticoagulant | 8/54 | 1/60 | 0.013 |
| Salicylic acid | 8/54 | 3/60 | 0.076 | |
| GIAD lesion characteristics | Multiple locations | 25/54 | 13/60 | 0.005 |
| Location: | ||||
| Cecum | 22/54 | 26/60 | 0.780 | |
| Right colon | 19/54 | 16/60 | 0.325 | |
| Transverse colon | 10/54 | 5/60 | 0.108 | |
| Left colon | 6/54 | 9/60 | 0.484 | |
| Sigmoid | 5/54 | 6/60 | 0.894 | |
| Rectum | 2/54 | 7/60 | 0.166 | |
| Fundus | 6/54 | 3/60 | 0.304 | |
| Antrum | 9/54 | 4/60 | 0.093 | |
| Bulb | 6/54 | 3/60 | 0.304 | |
| Duodenum | 14/54 | 6/60 | 0.026 | |
| Jejunum | 5/54 | 1/60 | 0.507 | |
| Ileum | 2/54 | 0/60 | 0.181 | |
| Total number of lesions ≥ 10 | ||||
| Number of lesions per segment ≥ 5 | 16/54 | 4/60 | <0.001 | |
| Cecum | 10/22 | 2/26 | 0.008 | |
| Right colon | 12/19 | 3/16 | 0.007 | |
| Transverse colon | 7/10 | 2/5 | 0.082 | |
| Left colon | 4/6 | 3/9 | 0.706 | |
| Sigmoid colon | 2/5 | 3/6 | 1 | |
| Rectum | 1/2 | 3/7 | 0.602 | |
| Fundus | 3/6 | 0/3 | 0.1 | |
| Antrum | 3/9 | 0/4 | 0.1 | |
| Bulb | 1/6 | 0/3 | 0.459 | |
| Duodenum | 1/14 | 1/6 | 1 | |
| Jejunum | 3/5 | 0/1 | 0.103 | |
| Ileum | 1/2 | 0/0 | 0.459 | |
| Size of lesions per segment ≥ 5 mm | ||||
| Cecum | 18/22 | 11/26 | 0.008 | |
| Right colon | 15/19 | 6/16 | 0.033 | |
| Transverse colon | 2/10 | 2/5 | 0.520 | |
| Left colon | 5/6 | 6/9 | 0.604 | |
| Sigmoid colon | 3/5 | 3/6 | 0.571 | |
| Rectum | 2/2 | 4/7 | 0.500 | |
| Fundus | 6/6 | 1/3 | 0.183 | |
| Antrum | 8/9 | 3/4 | 1 | |
| Bulb | 5/6 | 2/3 | 1 | |
| Duodenum | 10/14 | 1/6 | 0.06 | |
| Jejunum | 3/5 | 1/1 | 1 | |
| Ileum | 2/2 | 0/0 | – | |
Risk factors for GIAD bleeding: multivariate study.
| Odds ratio (95% confidence interval) | p value | |
|---|---|---|
| Age ≥ 75 years | 3.495 (1.389-8.797) | 0.008 |
| Number of lesions ≥ 10 | 8.675 (2.260-33.304) | 0.002 |
| Chronic kidney disease | 8.043 (1.470-43.994) | 0.016 |
| Coronary artery disease | 4.198 (1.076-16.374) | 0.039 |
| Diabetes mellitus | 3.663 (1.013-13.252) | 0.048 |
In the present study, we evaluated the clinical features of GIAD in a Tunisian population and identified the following features as independent risk factors promoting GIAD bleeding: increased age, CKD, coronary artery disease, diabetes mellitus, and having ≥ 10 lesions. The natural history of GIAD is not fully understood. Nevertheless, the fact that all lesions have the potential to bleed and that incidental lesions are less likely to bleed is universally accepted.2 As for symptomatic GIAD lesions, clinical presentation varies from occult bleeding to visible bleeding (melena, hematemesis, hematochezia with or without hemodynamic instability). In our study, approximately half our patients (52.6%) had incidental GIAD lesions. In the other cases, melena was the most common symptom, followed by hypochromic anemia, hematemesis, and hematochezia. Those results corroborate the known epidemiology of GIAD lesions.4–6
In our study, the majority of the GIAD lesions were found in the colon (69.2%), followed by the small bowel (32.5%) and the stomach (19.3%). Such distribution was thought to be the most frequent, before the era of small bowel enteroscopy and video capsule endoscopy. In fact, it was once believed that GIAD lesions were mainly located in the cecum and the right colon, and to a lesser degree, in the stomach and the small bowel. However, recent studies have shown the opposite order to be true. In their study on patients with GIAD that underwent endoscopic examination of the entire GI tract, Neu et al. found that the small bowel was the most commonly involved location.7 The same distribution pattern was described by DeBedet et al. and Bollinger et al.8,9 Notably, even the distribution of angiodysplasia in the colon is controversial. The right colon is reported to be the most common colonic location in Western studies, whereas Japanese and Taiwanese studies have reported that colonic angiodysplasia is predominantly located in the left colon.5 In our study, the most frequent colonic segmental location was the cecum (56%), followed by the right colon (25.3%), the left colon (8%), the transverse colon (5.3%), and finally the rectum (1.3%).
Currently, no treatment guidelines are available for GIAD lesions, which explains the high variability in its management. This problem was highlighted by a 2015 Dutch study that applied a national web-based survey, to assess the treatment approach to GIAD by gastroenterologists.10 In current practice, clinicians tend to defer treatment of symptomatic GIAD, when no stigmata of hemorrhage are found, and most authors agree upon not treating incidental lesions. Thus, the identification of risk factors for GIAD bleeding is of paramount importance, to better understand the mechanisms and factors that cause a GIAD lesion to bleed, and consequently aid endoscopists in identifying high-risk groups, in which bleeding can be anticipated, predicted, and considered, even in the absence of stigmata of hemorrhage.
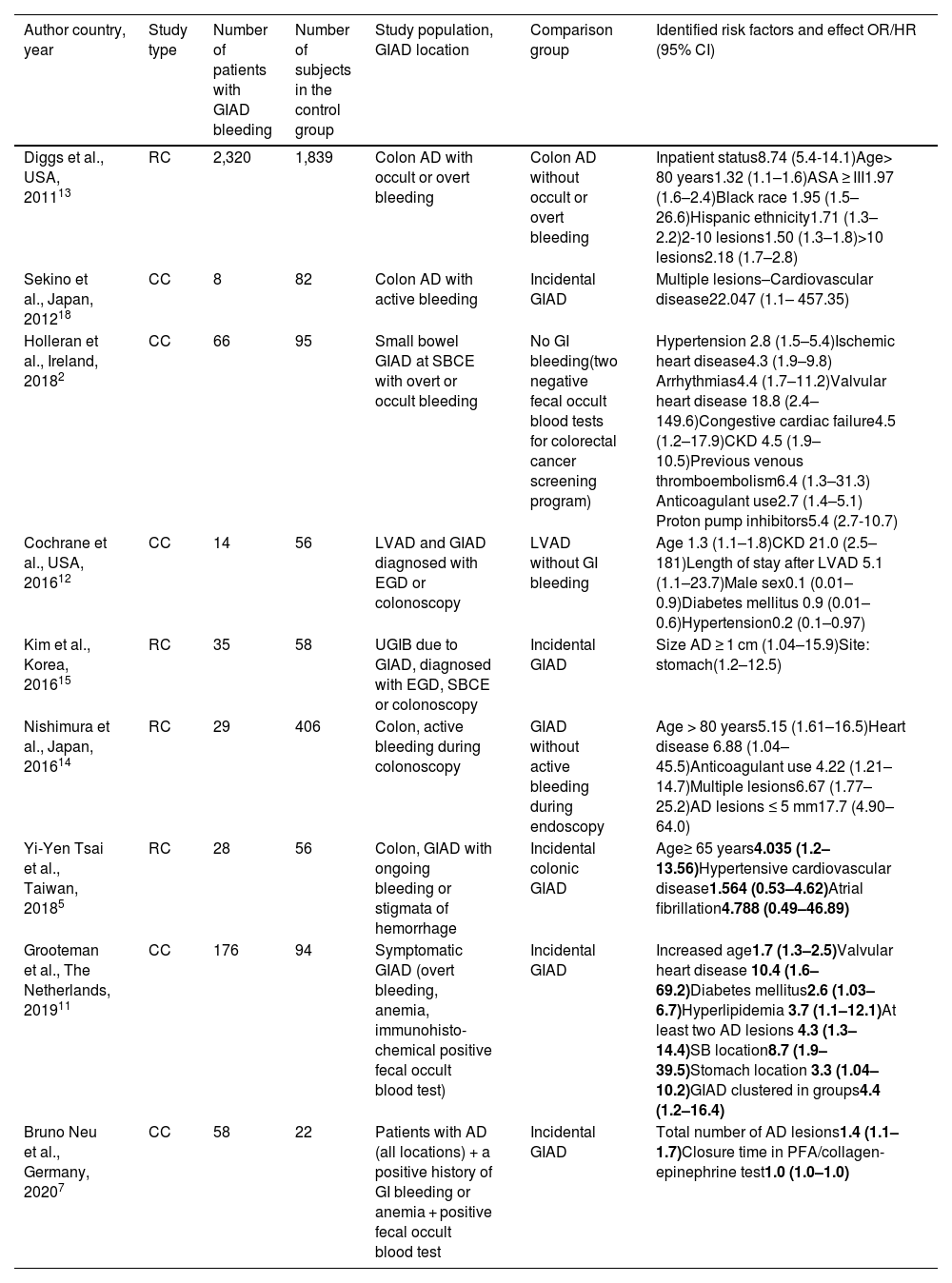
Only 2 years ago, in what is still a new area of research, the difference in risk factors between incidental and symptomatic angiodysplasia was highlighted in a case-control study, using a random sample from the general population as a control group.11 The results of that study led the authors to conclude that incidental and symptomatic GIAD lesions might be different disease states. The currently available studies on risk factors for GIAD bleeding are summarized in Table 5. However, those studies are very heterogeneous, as some of them include only one location, and different control groups are used. Unfortunately, studies whose control groups are not made up of patients with incidental GIAD lesions do not allow us to distinguish specific factors that could be used to adequately differentiate incidental from symptomatic GIAD lesions during endoscopy.
Studies assessing risk factors for angiodysplasia-related bleeding.
| Author country, year | Study type | Number of patients with GIAD bleeding | Number of subjects in the control group | Study population, GIAD location | Comparison group | Identified risk factors and effect OR/HR (95% CI) |
|---|---|---|---|---|---|---|
| Diggs et al., USA, 201113 | RC | 2,320 | 1,839 | Colon AD with occult or overt bleeding | Colon AD without occult or overt bleeding | Inpatient status8.74 (5.4-14.1)Age> 80 years1.32 (1.1–1.6)ASA ≥ III1.97 (1.6–2.4)Black race 1.95 (1.5–26.6)Hispanic ethnicity1.71 (1.3–2.2)2-10 lesions1.50 (1.3–1.8)>10 lesions2.18 (1.7–2.8) |
| Sekino et al., Japan, 201218 | CC | 8 | 82 | Colon AD with active bleeding | Incidental GIAD | Multiple lesions–Cardiovascular disease22.047 (1.1– 457.35) |
| Holleran et al., Ireland, 20182 | CC | 66 | 95 | Small bowel GIAD at SBCE with overt or occult bleeding | No GI bleeding(two negative fecal occult blood tests for colorectal cancer screening program) | Hypertension 2.8 (1.5–5.4)Ischemic heart disease4.3 (1.9–9.8) Arrhythmias4.4 (1.7–11.2)Valvular heart disease 18.8 (2.4– 149.6)Congestive cardiac failure4.5 (1.2–17.9)CKD 4.5 (1.9–10.5)Previous venous thromboembolism6.4 (1.3–31.3) Anticoagulant use2.7 (1.4–5.1) Proton pump inhibitors5.4 (2.7-10.7) |
| Cochrane et al., USA, 201612 | CC | 14 | 56 | LVAD and GIAD diagnosed with EGD or colonoscopy | LVAD without GI bleeding | Age 1.3 (1.1–1.8)CKD 21.0 (2.5–181)Length of stay after LVAD 5.1 (1.1–23.7)Male sex0.1 (0.01–0.9)Diabetes mellitus 0.9 (0.01–0.6)Hypertension0.2 (0.1–0.97) |
| Kim et al., Korea, 201615 | RC | 35 | 58 | UGIB due to GIAD, diagnosed with EGD, SBCE or colonoscopy | Incidental GIAD | Size AD ≥ 1 cm (1.04–15.9)Site: stomach(1.2–12.5) |
| Nishimura et al., Japan, 201614 | RC | 29 | 406 | Colon, active bleeding during colonoscopy | GIAD without active bleeding during endoscopy | Age > 80 years5.15 (1.61–16.5)Heart disease 6.88 (1.04–45.5)Anticoagulant use 4.22 (1.21–14.7)Multiple lesions6.67 (1.77–25.2)AD lesions ≤ 5 mm17.7 (4.90–64.0) |
| Yi-Yen Tsai et al., Taiwan, 20185 | RC | 28 | 56 | Colon, GIAD with ongoing bleeding or stigmata of hemorrhage | Incidental colonic GIAD | Age≥ 65 years4.035 (1.2–13.56)Hypertensive cardiovascular disease1.564 (0.53–4.62)Atrial fibrillation4.788 (0.49–46.89) |
| Grooteman et al., The Netherlands, 201911 | CC | 176 | 94 | Symptomatic GIAD (overt bleeding, anemia, immunohisto-chemical positive fecal occult blood test) | Incidental GIAD | Increased age1.7 (1.3–2.5)Valvular heart disease 10.4 (1.6–69.2)Diabetes mellitus2.6 (1.03–6.7)Hyperlipidemia 3.7 (1.1–12.1)At least two AD lesions 4.3 (1.3–14.4)SB location8.7 (1.9–39.5)Stomach location 3.3 (1.04–10.2)GIAD clustered in groups4.4 (1.2–16.4) |
| Bruno Neu et al., Germany, 20207 | CC | 58 | 22 | Patients with AD (all locations) + a positive history of GI bleeding or anemia + positive fecal occult blood test | Incidental GIAD | Total number of AD lesions1.4 (1.1–1.7)Closure time in PFA/collagen-epinephrine test1.0 (1.0–1.0) |
AD: angiodysplasia; CC: case-control study; CI: confidence interval; CKD: chronic kidney disease; EGD: esophagogastroduodenoscopy; HR: hazard ratio; LVAD: left ventricular assist device; OR: odds ratio; RC: retrospective cohort; SB: small bowel; SBCE: small bowel capsule endoscopy; UGIB: upper gastrointestinal bleeding.
In our study, age above 75 years was identified as an independent risk factor for GIAD bleeding. Increased age is one of the most commonly identified risk factors for bleeding,5,11,12 albeit the reason for this is still unknown. In 2020, Neu et al. speculated that age might merely be a confounding factor, regarding the number of lesions and other comorbidities,7 but they have established an exponential dependency of increasing age on an increasing number of GIAD lesions. Therefore, we made sure to look for an association between the presence of multiple lesions and increased age (p = 0.92) and to adjust for age in the multivariate analysis. Another commonly accepted risk factor is the total number of lesions. In our study, ≥ 10 lesions was identified as an independent risk factor for GIAD bleeding. Those results are in accordance with the current literature. In fact, Nishimura et al. and Diggs et al. identified multiple lesions as a risk factor for active bleeding in the colon.13,14 In addition, Neu et al. identified the total number of lesions in the entire GI tract as an independent factor for GIAD bleeding.7 Based on those findings, several authors suggest exploring the entire GI tract, when an angiodysplasia lesion is diagnosed, even if it was incidentally found.
In our study, we found no association between any of the GI locations and the occurrence of GIAD-related bleeding. In fact, it is accepted that all GIAD lesions can bleed at some point, regardless of their location. However, whether certain locations themselves are associated with a higher risk of bleeding is still unknown. A study analyzing the risk factors of angiodysplasia presenting as upper GI bleeding found that non-antral locations (gastric body/fundus) were associated with GI bleeding.15 Those findings are in contrast with a classic theory suggesting that the antrum is more predisposed to venous obstruction and vascular ectasia than other segments of the stomach due to the vigor of the muscular contraction in the antrum. It is not yet known if the same theory could be proposed for gastric angiodysplasia, and even if it could, it would only explain a greater number of lesions in the antrum, and not the predisposition to bleeding.16
Regarding the distribution in the colon, Nishimura et al. found that actively bleeding angiodysplasia lesions were located mainly in the right colon,14 but with no statistical significance in the multivariate analysis. The right-sided location was also identified by Diggs et al. as an independent predictive factor of endoscopic therapy requirement.13 In contrast, those associations were not found in more recent studies.5,7 We believe that no conclusions can be drawn from the current literature concerning the possible role of GIAD size and location in the occurrence of bleeding. In our study, CKD, coronary artery disease, and diabetes mellitus were identified as independent risk factors for GIAD bleeding. Those results concur with the published literature.
Several studies have shown that CKD patients, whether undergoing hemodialysis or not, are prone to GI bleeding, regardless of the underlying cause. This can be explained by the accumulation of uremic toxins, which impairs platelet aggregation and adhesion.17 In contrast to our findings, in which hemodialysis was not identified as a risk factor for GIAD bleeding in the multivariate analysis, the risk of bleeding in patients with CKD appears to be even higher in patients undergoing hemodialysis. A recent Taiwanese nation-wide population-based cohort study investigating the impact of CKD on the incidence of lower GI bleeding found a higher incidence of angiodysplasia-related bleeding in CKD patients undergoing dialysis, when compared with dialysis-free patients and control subjects.17
Coronary artery disease was associated with GIAD-related bleeding in several studies.13,14,18 The underlying mechanism is not yet known. A possible explanation could be the wide use of antiplatelets in that population. In a prospective case-control study, Neu et al. demonstrated that the alterations in primary hemostasis that are typically observed in patients on salicylic acid are associated with an increased risk of GIAD-related bleeding.7 Indeed, antiplatelets can increase the risk of GIAD bleeding by irreversibly inhibiting platelets. This can possibly occur in the upper GI tract, by reducing prostaglandin synthesis, which can then expose the gastric mucosa to a higher level of acidity. On the other hand, in a retrospective cohort study by Cochrane et al. that primarily aimed to compare the rate of GIAD bleeding in patients with a continuous-flow left ventricular assist device (LVAD) with other causes of bleeding, those authors found that patients on proton pump inhibitors were 14-times more likely to have GI bleeding, regardless of the underlying bleeding cause.12 Those findings obviously contradict the antiplatelet and acidity theory. However, patients with LVAD form a very specific group, in which ischemic mechanisms are more amplified and probably account for the occurrence of GIAD hemorrhage. Thus, we consider that further evaluation of the role of antiplatelets on a larger and less specific population is still needed.
Strengths and limitationsDespite the extensive data we collected from an 11-year-old database, some limitations must be acknowledged. First, ours is a single center retrospective study reflecting current clinical practice, and therefore, a standard management for all patients cannot be guaranteed. Second, 64.9% of our patients underwent either lower or upper endoscopy, and none of the patients presenting with obscure GI bleeding had a complete endoscopic assessment because video capsule endoscopy is not available in public hospitals in Tunisia, nor is it covered by the National Health Insurance Fund. Lastly, we did not determine the risk factors for GIAD lesions, as we did not have a control group. In spite of those limitations, we believe that our study has several strengths. First, the identification of different risk factors was based on a relatively homogeneous enrollment criterion and a thorough analysis of a large database, enabling us to separate and compare the GIAD lesions based on their distribution, number, size, and clinical outcomes. Second, our study sheds light on the importance of establishing a risk stratification-based management of GIAD lesions and actively contributes to the assessment and understanding of the substantial role of individual risk factors in the clinical picture.
ConclusionIn the present study, we identified increased age, a higher number of lesions, and the comorbidities of CKD, diabetes mellitus, and coronary artery disease as independent predictors of GIAD bleeding. Based on those results and our review of the current literature, we strongly believe that patients with incidental GIAD lesions and multiple risk factors for GIAD bleeding should be treated and will benefit from further screening for other lesions throughout the GI tract. However, further studies analyzing the different risk profiles and the efficacy of different treatments are still needed.
Financial disclosureNo financial support was received in relation to this article.
Conflict of interestThe authors declare that there is no conflict of interest.
Author contributionsDr. Nasr and Dr. Khsiba: conception and design.
Dr. Mahmoudi and Dr. Hamzaoui: analysis and interpretation of the data.
Dr. Ben Mohamed and Dr. Yaakoubi: drafting of the article.
Dr. Medhioub: critical revision of the article for important intellectual content.
Dr. Azouz: final approval of the article.