Thromboembolic events (TEEs) have been documented in patients with inflammatory bowel disease (IBD). Incidence in hospitalized pediatric patients with IBD is 117.9/10,000, with a relative risk of 2.36 (95% confidence interval: 2.15-2.58). An estimated 1.3 to 6.4% of adults and 3.3% of children with IBD develop cerebrovascular complications during the course of the disease, and they are more frequent during exacerbations.1–3
A male child seen at 2 years and 7 months of age, with no history of IBD or autoimmune disease, presented with lower gaastrointestinal bleeding, diarrhea, and nocturnal bowel movements starting at 14 months of age. Infections, allergies, and primary and secondary immunodeficiency were ruled out as causes. ANCA and ASCA antibodies were negative. Colonoscopy revealed a hyperemic, friable, and nodular cecal mucosa, as well as micro-ulcers located predominantly in the sigmoid colon and rectum. The histology study was consistent with IBD and immunohistochemistry was negative for Epstein-Barr virus and cytomegalovirus. The child was treated with 2 mg/kg/day of prednisone, 1 mg/kg/day of azathioprine, and due to failed remission, infliximab at a dose of 5 mg/kg.
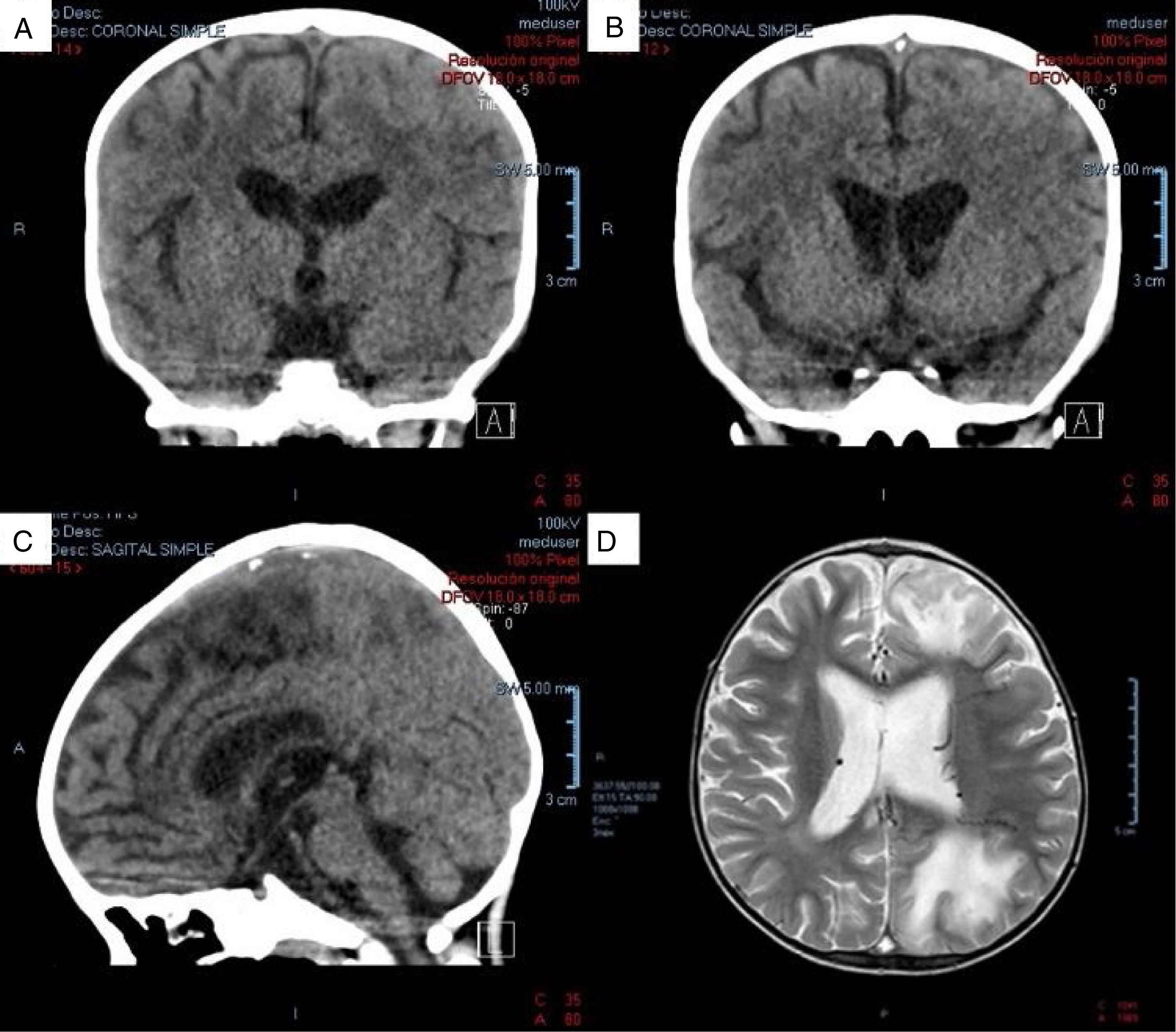
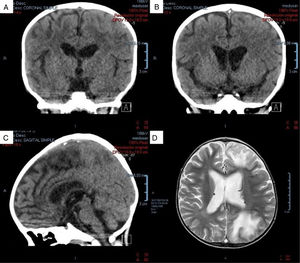
At one month of hospitalization, the patient presented with two events of tonic-clonic focal onset motor seizures, located in the left hemibody and lasting for 1 min, without altering consciousness. Neurologic examination revealed normal cranial pairs, preserved muscle tone, 4/5 overall muscle strength, right ++/++++ left +++/++++ superior muscle stretch reflex, inferior muscles with bilateral exhaustible clonus, normal superficial sensitivity, negative Babinski reflex, and negative cerebellar, meningeal, and neurocutaneous signs. Table 1 shows the laboratory results at the time of the event. Computed axial tomography and nuclear magnetic resonance imaging of the head identified venous sinus thrombosis in the entire tract of the superior sagittal sinus and in two confluent veins of the frontal region, bilaterally. The cerebral parenchyma had zones of venous infarct in the frontal and left parietal-occipital regions, as well (Fig. 1). Normal homozygous MTHFR A1298C, heterozygous MTHFR C677 T, homozygous prothrombin G20210A, and homozygous Leiden factor V G1691A results ruled out primary thrombophilia, as did normal homocysteine, C protein, S protein, and antithrombin III values. An echocardiogram showed no images suggestive of thrombi or vegetations in the large vessels, valves, or cardiac cavities.
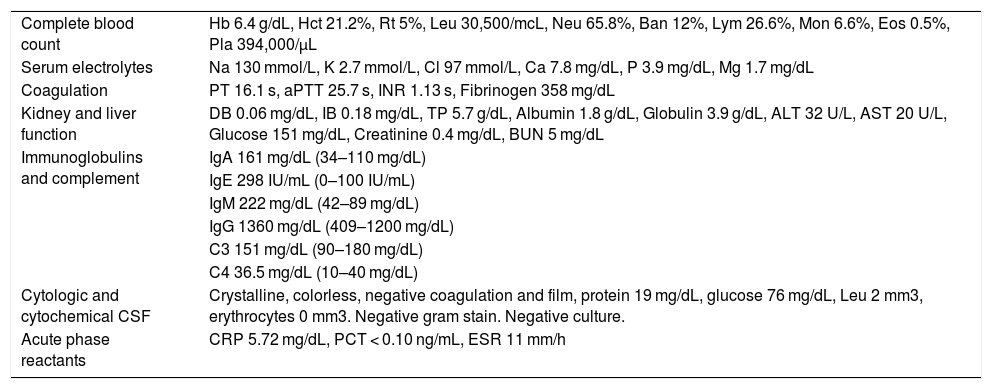
Laboratory studies.
| Complete blood count | Hb 6.4 g/dL, Hct 21.2%, Rt 5%, Leu 30,500/mcL, Neu 65.8%, Ban 12%, Lym 26.6%, Mon 6.6%, Eos 0.5%, Pla 394,000/μL |
| Serum electrolytes | Na 130 mmol/L, K 2.7 mmol/L, Cl 97 mmol/L, Ca 7.8 mg/dL, P 3.9 mg/dL, Mg 1.7 mg/dL |
| Coagulation | PT 16.1 s, aPTT 25.7 s, INR 1.13 s, Fibrinogen 358 mg/dL |
| Kidney and liver function | DB 0.06 mg/dL, IB 0.18 mg/dL, TP 5.7 g/dL, Albumin 1.8 g/dL, Globulin 3.9 g/dL, ALT 32 U/L, AST 20 U/L, Glucose 151 mg/dL, Creatinine 0.4 mg/dL, BUN 5 mg/dL |
| Immunoglobulins and complement | IgA 161 mg/dL (34–110 mg/dL) |
| IgE 298 IU/mL (0–100 IU/mL) | |
| IgM 222 mg/dL (42–89 mg/dL) | |
| IgG 1360 mg/dL (409–1200 mg/dL) | |
| C3 151 mg/dL (90–180 mg/dL) | |
| C4 36.5 mg/dL (10–40 mg/dL) | |
| Cytologic and cytochemical CSF | Crystalline, colorless, negative coagulation and film, protein 19 mg/dL, glucose 76 mg/dL, Leu 2 mm3, erythrocytes 0 mm3. Negative gram stain. Negative culture. |
| Acute phase reactants | CRP 5.72 mg/dL, PCT < 0.10 ng/mL, ESR 11 mm/h |
ALT: alanine aminotransferase; aPTT: activated partial thromboplastin time; AST: aspartate aminotransferase; Ban: bands; BUN: blood urea nitrogen; C: complement; Ca: calcium; Cl: chloride; CRP: C-reactive protein; CSF: cerebrospinal fluid; DB: direct bilirubin; Eos: eosinophils; ESR: erythrocyte sedimentation rate; Hb: hemoglobin; Hct: hematocrit; IB: indirect bilirubin; Ig: immunoglobulin; INR: international normalized ratio; K: potassium; Leu: leukocytes; Lym: lymphocytes; Mg: magnesium; Mon: monocytes; Na: sodium; Neu: neutrophils; P: phosphorus; PCT: procalcitonin; Pla: platelets; PT: prothrombin time; Rt: reticulocytes; s: seconds; TP: total proteins.
A) Coronal view of non-contrast-enhanced tomography scan. Hypodense zones are in the superior sagittal sinus (hypodense triangle sign). B) Coronal view of non-contrast-enhanced tomography scan. In a posterior slice, hyperdense zones alternate, supporting the diagnosis of cerebral venous sinus thrombosis in the superior sagittal sinus. C) Sagittal view of non-contrast-enhanced tomography scan. Hyperdense zones at the level of the superior sagittal sinus (venous sinus thrombosis). D) Magnetic resonance image of the encephalon, axial view in phase T2. Zones of venous infarct in the frontal and left parietal-occipital regions.
Treatment began with 2 mg/kg/day of subcutaneous enoxaparin and 20 mg/kg/day of levetiracetam but was suspended after 10 days due to increased disease activity and active bleeding.
The patient was released four months later, completely recovered and with no neurologic sequelae. A specific speech disorder was detected at outpatient follow-up.
Ever since the reported association between cerebral venous thrombosis and ulcerative colitis, pediatric case series have been documented that report the superior sagittal sinus as the most common site of venous thrombosis in the brain.4,5
Thrombosis pathophysiology in IBD is multifactorial: thrombocytosis/platelet activation, hyperhomocysteinemia, high fibrinogen levels, impaired fibrinolysis, autoantibodies, increased procoagulation factors, decreased anticoagulation factors, and procoagulation mutations.6 Coagulation activity in IBD is related to the activity and colonic extension of the disease.7 Anemia has been documented in 49% of cases with thrombosis, thrombocytosis in 26%, and no identifiable cause in up to 14%, as could be the case in our patient.7
In children with the first TEE, nonactive IBD, and an unrelated reversible triggering factor (immobilization, recent surgery, trauma, oral anticontraceptive use, or catheter), the Canadian Association of Gastroenterology recommends the use of anticoagulant therapy for 3 months, until the risk factor has been resolved for one month. In the context of active disease, the suggestion is to continue treatment until there have been 3 months of remission.8
Routine thromboprophylaxis is not routinely recommended for hospitalized children with IBD relapse that have no previous history of thrombosis. However, in a review of pediatric IBD cases, there was a 1% incidence of venous TEEs and the risk factors of older age, central venous catheter, parenteral nutrition, and hypercoagulability were identified, suggesting a possible benefit in relation to primary pharmacologic thromboprophylaxis. However, the possible side effects of intracranial bleeding and increased gastrointestinal bleeding must be kept in mind.3,6
Thromboprophylaxis is recommended in patients considered high-risk, such as hospitalized patients, those with colonic involvement, patients with a personal history of venous thromboembolism that will undergo surgery, patients with a family history of venous thromboembolism in a first-degree relative, known thrombophilia, persistent antiphospholipid antibodies, oral contraceptive use, thalidomide use, smoking, obesity, or a central venous catheter.9
Low-molecular-weight heparin is recommended in children as follows: in those that weigh less than 60 kg, 0.5 mg/kg twice a day, subcutaneously, and in those that weigh more than 60 kg, subcutaneous administration of 30 mg twice a day or 40 mg once a day.3
Thrombotic status should be monitored, and risk factors identified, as an integral part of IBD treatment.
Ethical considerationsThe authors declare that the present article contains no personal information enabling the identification of the patient, who remains completely anonymous. Because the article is based on the review of a case record, no authorization by the hospital ethics committee was required.
Financial disclosureNo specific grants were received from public sector agencies, the business sector, or non-profit organizations in relation to this article.
Conflict of interestThe authors declare that there is no conflict of interest.
The authors wish to thank Dr. Judmila López Sánchez at the Department of Neurology of the Hospital Infantil de México Federico Gómez, SSA, Mexico City, Mexico.
Please cite this article as: Rivera-Suazo Y, Argüello Calderon I, Vázquez-Frías R. Trombosis del seno venoso sagital superior en paciente pediátrico con enfermedad inflamatoria intestinal: reporte de caso. Revista de Gastroenterología de México. 2020;85:364–366.