Celiac disease (CD) is an autoimmune enteropathy induced by dietary wheat gluten that can have serious consequences if not diagnosed and treated early. It is important to be familiar with other alterations associated with gluten ingestion due to the multiplicity of clinical presentations.
ObjectivesTo describe the most common CD presentation patterns and alterations associated with gluten in children from the northwest region of Mexico, with an incipient knowledge of its prevalence.
Patients and methodsAge, sex, family history, and gastrointestinal and extraintestinal symptoms were recorded in 24 patients within the time frame of 2006 to 2010. Biochemical and hematologic data were collected. Anti-gliadin and anti-transglutaminase antibodies were analyzed in all the cases, and haplotypes (HLA-DQ2/DQ8) and duodenal biopsy were evaluated in some of the cases.
ResultsOf the 24 patients (14 girls and 10 boys), 13 presented with typical CD with symptoms of poor gastrointestinal absorption; 7 patients with a mean age of 5 years presented with atypical CD; 2 had disease onset with gastrointestinal and extraintestinal (neurologic) problems; and 2 with other gluten-related disorders. All of the patients had positive serology; 11/15 presented with HLA-DQ2/DQ8 and 4 with at least one allele; damaged mucosa was observed in the 6 biopsies taken. A third of the patients were anemic, 6 presented with an albumin value of<3.5g/dL, and 4 with mineral deficiencies. A total of 83% of the patients improved with a gluten-free diet.
ConclusionsThe presentation patterns were: 1) typical CD, 2) atypical CD, 3) CD with gastrointestinal and extraintestinal (neurologic) symptoms, and 4) gluten-related disorders other than CD.
La enfermedad celiaca (EC) es una enteropatía autoinmune inducida por el gluten del trigo dietético, con serias consecuencias si no se diagnostica y trata tempranamente. Hay además otras alteraciones asociadas a la ingestión de gluten, que es importante conocer, por su multiplicidad de presentaciones clínicas.
ObjetivosDescribir los patrones más comunes de presentación de EC y alteraciones asociadas al gluten en niños de la región noroeste de México, con incipiente conocimiento de su prevalencia.
Pacientes y métodosSe registraron la edad, el género, la historia familiar y los síntomas gastro y extraintestinales, en 24 pacientes, entre 2006 y 2010. Se recogieron datos bioquímicos y hematológicos. Se analizaron anticuerpos antigliadinas y antitransglutaminasa en todos los casos; haplotipos (HLA-DQ2/DQ8) y biopsia duodenal en parte de los mismos.
ResultadosDe los 24 pacientes (14 mujeres y 10 varones), 13 presentaron EC típica con síntomas de mala absorción gastrointestinal; 7 promediando 5 años de edad, con EC no típica; 2 iniciaron con problemas gastro y extraintestinales (neurológicos), y 2 con otros desórdenes asociados al gluten. Todos presentaron serología positiva; 11/15 presentaron HLA-DQ2/DQ8 y 4 al menos un alelo; las 6 biopsias tomadas, mostraron mucosa dañada. Una tercera parte estaban anémicos, 6 con albúmina < 3.5g/dL, 4 con deficiencias de minerales. El 83% de los pacientes mejoró con la dieta sin gluten.
ConclusionesLos patrones de presentación fueron: 1) EC típica; 2) EC no típica; 3) EC con síntomas gastro y extraintestinales (neurológicos), y 4) sin EC, con otros desórdenes relacionados con el gluten.
Celiac disease (CD) is systemic, it is mediated immunologically, precipitated by exposure to dietary gluten, and it develops in genetically susceptible individuals. It is characterized by diverse clinical manifestations, specific antibodies, haplotypes HLA-DQ2 and DQ8, and enteropathy.1,2
CD presents with a wide variety of non-specific signs and symptoms that can be gastrointestinal or extraintestinal and thus has been classified as typical, atypical, asymptomatic, subclinical, and potential.2–4 Typical pediatric CD is characterized by delayed growth, diarrhea, emaciation, loss of appetite, and abdominal bloating; when it appears with any other sign or symptom it is atypical. Asymptomatic, or silent, CD does not present with clinically suspicious signs or symptoms, and is subclinical when it is below the threshold of clinical detection. Potential CD, also called latent CD, is defined by the presence of compatible antibodies and haplotypes, but with no abnormalities in the duodenal mucosa; it may or may not present with symptoms or enteropathy.1,2
There is also a recently recognized entity called non-celiac sensitivity or hypersensitivity to gluten.2,5 It is characterized by clinical symptoms (gastrointestinal) that are very similar to those of CD, the patients present with positive anti-gliadin antibody titers, but negative anti-transglutaminase antibody titers.5 They do not present with atrophy of the intestinal villi, but they do present with eosinophil infiltration into the duodenal and colonic mucosa; in addition, they do not present with allergies linked to IgE. In many cases of hypersensitivity, the patients have the HLA-DQ2 and DQ8 haplotypes, and in all cases their symptoms are resolved by a gluten-free diet.
Refractory CD is also well characterized and is defined by its symptoms of persistent or recurrent malabsorption and atrophy of the intestinal villi, despite a strict gluten-free diet during 6-12 months.2,6 Refractory CD can be type 1 or type 2. In type 1, the patients do not respond to a gluten-free diet, but their intraepithelial lymphocytes are normal. Type 2 is characterized by clones of abnormal intraepithelial lymphocytes that do not present with the CD3 and CD8 markers and the T-cell receptor, but they express CD3 intracellularly. It is associated with poor outcome due to the fact that it can progress to T-cell lymphoma.6,7
With this diversity of signs and symptoms, the diagnosis of CD and other gluten-associated alterations tends to be complicated, obscuring the dimension of the problem. It is estimated that between 1:100 and 1:200 individuals of any given population suffer from some form of CD; however, there are differences among the published data. For example, for the Mexican population, there was a 1:37 prevalence of the IgA anti-transglutaminase antibody resulting from the analysis of serum from healthy donors;8 when the serum was reanalyzed for anti-endomysial antibodies, prevalence decreased to 1:1689. In contrast, in a recent study conducted on 7,798 persons in the United States, 1:141 were positive for celiac disease, whereas among 1,686 Mexican Americans in the same study using the same serologic indicators, not a single individual was positive.10
The consequences of CD on health, if it is not diagnosed and treated early on, can be very severe, especially in children, because it affects their growth and development. The diagnosis and treatment of CD is recent in Mexico. Therefore, the aim of this study was to describe the most common presentation patterns of CD and other gluten-associated alterations in children from the northwest region of Mexico, with an incipient knowledge of its prevalence.
Patients and methodsChildren and adolescents participated in the study after their parents gave their informed consent. Within the time frame of 2006 and 2010, 47 patients with clinical suspicion of CD were sequentially included in the study. Twenty-three of them were excluded because they had negative serologic markers and/or haplotypes or because they presented with some other pathology. Thus, a total of 24 patients were selected for the study. The variables taken into account were: age, sex, family history, and the existence of diseases such as type 1 diabetes mellitus, thyroiditis, osteoporosis, Down's syndrome, peripheral neuropathy, ataxia, and behavioral disorders. The symptoms regarded as typical in accordance with the Oslo definitions were chronic diarrhea, abdominal bloating, weight loss or growth delay, loss of appetite, and emaciation.2 Symptoms were considered atypical when they were isolated gastrointestinal ones, such as gastroesophageal reflux, or if the patient did not present with malabsorption, such as constipation and abdominal pain;2 extraintestinal symptoms, such as anemia, neuropathy, fatigue, and low bone density were also included.1,2
The laboratory work-up was full blood count, serum iron, creatinine urea, glucose, calcium, albumin, the stool ova and parasite test, and in some cases the D-xylose absorption test in blood or urine. The specific serologic tests of IgG and IgA anti-gliadin antibodies were done, as well as IgA anti-transglutaminase through the enzyme linked immunosorbent assay (ELISA), as previously performed.11 The cut-off point was the mean+2 SD of the optic density of 30 serum samples from healthy children. Reactivity was expressed as an index; in other words, the optic density of the serum to be tested divided by the value of the cut-off point. The indices equal to or greater than 1.0 were regarded as positive.12 Low titers were between 1 and 5, moderate between 5 and 10, high between 10 and 20, and very high was greater than 20. In 15 children DNA extracted from the total blood was analyzed along with the HLA-DQ2 and HLA-DQ8 haplotypes by conventional PCR, using the indicators designed by Olerup et al.13 In 6 cases, intestinal mucosa biopsy was taken with a peroral capsule or through endoscopy, and analyzed.
Dietary follow-up was carried out with the aid of a dietary guide for children with celiac disease that were between the ages of one and 6.14 Likewise, the specific antibodies continued to be monitored during follow-up until they were negative.
The study project was approved by the ethics committees of the Hospital Infantil de Estado de Sonora and the Centro de Investigación en Alimentación y Desarrollo, A.C.
The study was a description of cases and the index values used for the ELISA tests were the average of at least 4 dilutions that were duplicated (8 absorbance readings).
ResultsOf the 24 patients, 14 were girls and 10 were boys. All except one, who at the beginning of the study was already on a gluten-free diet, were positive for IgA and IgG anti-gliadin antibodies. That same case and two that were under 2 years of age, were negative for IgA anti-transglutaminase; the rest of the children presented with positive indices. Four of the patients presented with very high specific antibody indices, 5 with high indices, 2 with moderate indices, and the remaining 13 with low indices. Of the 15 children in whom genetic predisposition was analyzed, 7 had HLA-DQ2, 4 had HLA-DQ8, and the remaining 4 had at least one allele of the two haplotypes. There were signs of malnutrition affecting development in 3 of the children that presented with underweight above 40%; 7 had underweight between 26 and 39%, and one child presented with underweight of 22%. In regard to minerals, 6 presented with less than 40 u/dL of serum iron and hemoglobin was under 10g/dL in 8 children; four presented with hypocalcemia.
Taking into account the signs and symptoms, the specific antibody indices, and in some cases, the haplotypes or biopsy, the most common presentation patterns in the sample were classified into 4 groups: 1) CD with a typical presentation, 2) CD with an atypical onset, 3) CD with gastrointestinal and extraintestinal symptoms (malabsorption and neurologic problems), and 4) non-celiac gluten-related disorders.
Table 1 presents 13 cases, 10 of which were children between 6 months and 3 years of age that had characteristics of typical CD development. In other words, they were small and presented with the sudden and severe gastrointestinal symptoms of malabsorption, malnutrition, and associated complications, shortly after gluten introduction.15,16 The 3 remaining cases were in this category due to their malabsorption problems2, even though gluten was not introduced into their diet until later in their lives.
Characteristics of cases with typical celiac disease.
| Case | Age/Sex | Signs and symptoms | Indexesa | Haplotypes/ biopsy | ||
| IgA anti-gliadins | IgG anti-gliadins | IgA anti-TG | ||||
| 1 | 3 y. F | Diarrhea, abdominal distension | 14.01 | 1.73 | 10.16 | NA |
| 2 | 9 m. M | Chronic diarrhea, malabsorption, grade 3 malnutrition | 37.68 | 5.64 | 36.70 | Marsh biopsy-2 |
| 3 | 3 y. M | Diarrhea, weight loss, recurrent abdominal pain | 10.01 | 3.10 | 12.29 | NA |
| 4 | 11 m. F | Diarrhea, abdominal distension | 14.76 | 3.60 | 11.33 | NA |
| 5 | 2 y. F | Intermittent chronic diarrhea, grade 2 malnutrition | 8.80 | 3.50 | 3.80 | NA |
| 6 | 2 y. M | Diarrhea, abdominal distension, retarded growth, grade 2 malnutrition | 21.40 | 4.10 | 13.13 | HLA-DQ2Marsh biopsy-3 |
| 7 | 2.9 y. M | Diarrhea and dehydration, grade 2 malnutrition, anemia, tetany, muscular hypotrophy, malnutrition, underheight for age | 35.70 | 3.50 | 26.80 | HLA-DQ8Marsh biopsy-3 |
| 8 | 6 m. M | Hypocalcemia, abdominal distension | 1.32 | 1.37 | 1.10 | DQA1*0301 |
| 9 | 11 m. F | Intermittent diarrhea for 3 months | 2.13 | 8.80 | 3.80 | NA |
| 10 | 8 y. F | Diarrhea, malabsorption, grade 1 malnutrition | 20.00 | 23.30 | 11.00 | HLA-DQ2DQA1*0301 |
| 11 | 8 y. F | Abdominal pain, chronic diarrhea | 23.30 | 1.00 | 9.70 | NA |
| 12 | 11 m. M | Diarrhea, grade 3 malnutrition, abdominal distension, emaciation, malabsorption syndrome | 2.79 | 1.85 | 1.26 | HLA-DQ2 |
| 13 | 5 y. F | Vomiting, diarrhea, grade 2 malnutrition | 1.16 | 1.40 | 1.01 | NA |
NA: not analyzed; TG: transglutaminase.
Among the most common signs and symptoms in the patients with typical CD in Table 1 were chronic diarrhea in 11/13 and abdominal bloating (5/13). This group presented with the highest antibody indices of more than 10 or 20 times the healthy reference value.
In relation to the follow-up of the gluten-free diet and its effects on nutritional status, all the children in the group presented in Table 1 responded adequately. Those that presented with some degree of malnutrition upon CD diagnosis, recovered the weight and height for their age with dietary treatment. Only in case 5, did the nutritional status recovery take several months after treatment.
Table 2 shows 7 cases of children from 15 months to 9 years of age, with a mean age of 6 years. They presented with isolated gastrointestinal symptoms at the onset of CD, as well as extraintestinal components as defined by Aurangzeb et al.15 Among their signs and symptoms were gastroesophageal reflux in 4/7 and abdominal pain in all 7. Those that presented with malnutrition also had a history of recurrent respiratory disease. None of these cases had high or very high antibody indices.
Characteristics of cases with atypical celiac disease onset.
| Case | Age/Sex | Signs and symptoms | Indexesa | Haplotypes/ biopsy | ||
| IgA anti-gliadins | IgG anti-gliadins | IgA anti-TG | ||||
| 14 | 1y. 3m. F | Onset at 7 months with constipation, with episodes of abdominal pain and diarrhea added at one year at one year | 1.03 | 1.05 | 0.57 | HLA-DQ2 |
| 15 | 2 y. F | Gastroesophageal reflux at 5 months of age, pain and abdominal distension, anemia and retarded growth at 9 months, grade 2 malnutrition, gluten-free diet since one year of age | Not evaluated at the beginning | Not evaluated at the beginning | Negative at 11 months of age | HLA-DQ2 DQA1*0301Marsh biopsy-2 |
| 16 | 9 y. F | Abdominal pain, bloating, hyporexia | 0.68 | 1.00 | 1.00 | DQB1*0201 |
| 17 | 1 y. 11m. F | Gastroesophageal reflux, recurrent abdominal pain, abdominal distension, recurrent respiratory infections, grade 2 malnutrition | 2.76 | 2.05 | 0.78 | HLA-DQ2Marsh biopsy-3 |
| 18 | 9 y. M | Chronic gastroesophageal reflux, abdominal pain | 1.66 | 1.75 | 2.11 | HLA-DQ8DQA1*0501 |
| 19 | 7 y. M | Abdominal pain and chronic gastroesophageal reflux, afterwards intermittent diarrhea | 3.49 | 2.43 | 2.10 | HLA-DQ8DQA1*0501 |
| 20 | 2 y. F | Bloating, abdominal pain | 4.80 | 2.67 | 2.90 | DQA1*0301 |
TG: transglutaminase.
In some of the patients in Table 2, there was no short-term disappearance of the symptoms once the gluten-free diet was begun; instead, it was gradual. The same held true for their nutritional status recovery, which was achieved several months after the dietary treatment. Case 15 did not recover the rate of growth until more than one year after treatment. Symptoms were reduced in case 18, but reflux did not completely disappear.
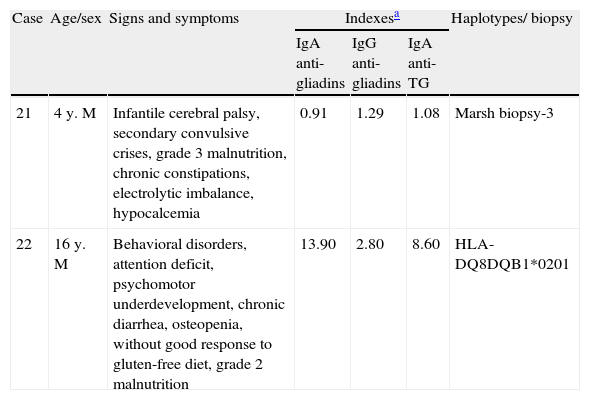
Table 3 shows 2 cases of children 4 and 16 years of age that could be classified as having atypical CD onset, but they were placed in a separate table because they both began the study with neurologic problems. One of the patients had behavioral and attention deficit disorders; the other presented with cerebral palsy. In both cases, the neurologic problems (extra-intestinal) presented together with the gastrointestinal symptoms - constipation in one of them and diarrhea in the other; and they both had advanced malnutrition. One of the cases had low titers, but positive specific antibodies, whereas they were between moderate and high in the other. There was extra evidence in both cases; a biopsy of the intestinal mucosa in one case and HLA-DQ8 detection in the other.
Characteristics of cases of celiac disease with gastro and extraintestinal symptoms.
| Case | Age/sex | Signs and symptoms | Indexesa | Haplotypes/ biopsy | ||
| IgA anti-gliadins | IgG anti-gliadins | IgA anti-TG | ||||
| 21 | 4 y. M | Infantile cerebral palsy, secondary convulsive crises, grade 3 malnutrition, chronic constipations, electrolytic imbalance, hypocalcemia | 0.91 | 1.29 | 1.08 | Marsh biopsy-3 |
| 22 | 16 y. M | Behavioral disorders, attention deficit, psychomotor underdevelopment, chronic diarrhea, osteopenia, without good response to gluten-free diet, grade 2 malnutrition | 13.90 | 2.80 | 8.60 | HLA-DQ8DQB1*0201 |
TG: transglutaminase.
The cases shown in Table 3 were difficult to follow in regard to symptom disappearance and nutritional status recovery. In fact, the youngest boy had partial recovery and the older boy had only partial symptom disappearance, but he did not have nutritional status recovery; they could be considered cases of refractory CD.
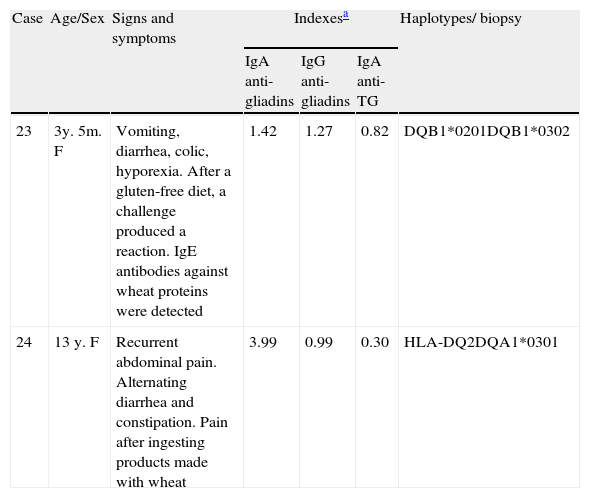
Table 4 presents 2 cases of gluten-related disorders; one, mediated by IgE, with an allergic response, and the other, mediated by IgA, with non-celiac gluten sensitivity, given that its IgA anti-transglutaminase antibody index was negative. The signs and symptoms in the two patients, one 3 years and 5 months old and the other 13 years old, were gastrointestinal and remitted with a gluten-free diet. After a gluten challenge, allergy mediated by IgE was demonstrated in the youngest girl; in the other girl non-celiac gluten sensitivity was suspected and she continued a gluten-free diet.
Characteristics of cases with other gluten-associated alterations.
| Case | Age/Sex | Signs and symptoms | Indexesa | Haplotypes/ biopsy | ||
| IgA anti-gliadins | IgG anti-gliadins | IgA anti-TG | ||||
| 23 | 3y. 5m. F | Vomiting, diarrhea, colic, hyporexia. After a gluten-free diet, a challenge produced a reaction. IgE antibodies against wheat proteins were detected | 1.42 | 1.27 | 0.82 | DQB1*0201DQB1*0302 |
| 24 | 13 y. F | Recurrent abdominal pain. Alternating diarrhea and constipation. Pain after ingesting products made with wheat | 3.99 | 0.99 | 0.30 | HLA-DQ2DQA1*0301 |
TG: transglutaminase.
For many years CD was a rare pathology in many countries, and Mexico was no exception. Nevertheless, in the last 15-20 years, its etiopathogenesis has become well understood and it is the most common inflammatory disease of the small bowel.16 It is an autoimmune disease that not only presents with exposure to gluten, but rather with a combination of other factors, including genetic predisposition and abnormalities in the structure of the small bowel.17 The procedures for the study and diagnosis of patients suspected of having CD have improved measurably.18,19
CD diagnostic criteria have changed as knowledge of its pathogenesis has increased and the techniques for its study have improved. Before its pathogenic mechanism was known, only its clinical symptoms were managed; tests were not very specific and the only way to confirm intestinal damage was through biopsy of the mucosa. In 1969 the European Society of Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) established the first diagnostic criterion. It consisted of taking three biopsies: one upon diagnosis, another after a gluten-free diet, and the third after a gluten challenge. In 1990 this criterion was changed to a single biopsy, together with serologic analysis and the clinical response to the gluten-free diet.20
Positive biopsy has been the criterion standard for CD diagnosis. However, over the last few years there has been a tendency to use the serologic analysis for making the definitive diagnosis. The importance and usefulness of this type of analysis is based not only on the knowledge of the presence of antibodies against the endogenous antigen, but also on a very important step in the pathogenic mechanism. In 1998, in two independent studies, Molberg et al.21 and van de Wal et al.22 showed that the tissue transglutaminase modified the gliadin peptides, increasing their reactivity for T-cells in CD. Thus, patients with CD not only present with antibodies against the gliadins, but also against their own transglutaminase.
Therefore, diagnosis focuses on the detection of IgA anti-gliadin, anti-endomysial, and anti-transglutaminase antibodies. The latter have also been used for evaluating CD incidence and prevalence in different populations.17 Due to the fact that 2% of the patients with CD are IgA-deficient, IgG is also quantified. Analyses of the anti-deamidated gliadin peptide antibodies are the most recent; that is, just as they would be found after proteolytic digestion and transglutaminase action for making them still more specific. There are reagent kits that simultaneously quantify IgA and IgG against those peptides.3,23
In very young children, as was the case with many of the patients in the present study, there is better sensitivity of IgA antibodies against gliadins than against transglutaminase or endomysium.24 Therefore, anti-gliadin antibody analysis is regarded as necessary in children<24 months old, with signs and symptoms suggestive of CD.23 Cases 14 and 17 in Table 2 show this very clearly; their indices of anti-gliadin antibodies were positive, whereas their indices against transglutaminase were negative. In fact, cases 15 and 17 showed clearly affected biopsies (Marsh 2 and 3), even though their anti-transglutaminase antibody indices were negative. When the clinical results, serologic analysis, and biopsy results are conflicting, HLA-DQ2 and DQ8 analysis can be helpful. If these haplotypes are not found, it is certain that there is no CD since almost all patients present with one or both haplotypes.1,16,25
According to the ESPAGHAN1, DQ2 and DQ8 haplotype analysis should first be carried out for diagnosing asymptomatic children suspected of having CD. Their absence indicates there is no need to continue ordering more studies to rule out CD. This procedure was followed for the cases in Table 2. Case 15 was a girl that from the age of 7 months presented with intestinal symptoms but no diarrhea. Anti-gliadin antibodies were not analyzed and her anti-TG antibodies were negative. A biopsy revealed moderate atrophy. In addition, she presented with HLA-DQ2; the gluten-free diet and a gluten challenge contributed to the CD diagnosis. On the other hand, cases 17, 18, and 19 presented with conspicuous gastroesophageal reflux, which is not among the most mentioned in the bibliography. The 3 patients presented with predisposing haplotypes. In an analysis of Canadian children with CD, 8% of them presented with reflux at the beginning of the study.26
Of the 15 cases analyzed for haplotypes, the majority presented with the DQA1*0501 and DQB1*0201 alleles or the HLA-DQ2.5 haplotype, that make up both alleles. Only 3 of the cases could be HLA-DQ2.2 (DQA1*0201 and DQB1*0202) and 4 were HLA-DQ8; this, despite the fact that in a population of newborn infants from Sonora, the proportion of DQ2 was almost equal to that of DQ8 (1.2:1). 27 According to different authors, DQ2.5 has a greater predisposition to CD than DQ2.2,28 given that it is found on the surface of the antigen-presenting cells, and so has a greater possibility to interact.4,25 HLA-DQ2 is also thought to have greater predisposition than HLA-DQ8, because its expressed molecule binds to a much wider range of peptides.29
The HLA-DQ2 and DQ8 haplotypes in the study population could also be important in deciding the type of serologic analysis to be carried out. Unlike HLA-DQ8 that can recognize native or deamidated gliadin peptides, the T-cells that are restricted to HLA-DQ2 have a marked preference for the deamidated ones.4 Therefore, the circulating antibodies could differ and perhaps it would not be necessary to analyze the deamidated gliadin peptide antibodies in a population with a preponderance of HLA-DQ8, such as those of the American Indians;30 nor would it be the most appropriate in very young children, since their immune response is directed against non-deamidated gliadins.31
Due to their confusing symptoms, several of the study patients were not diagnosed with CD at the onset of the disease. This happens everywhere in the world, even in the developed countries that have recognized CD for many years. In adults, diagnosis is commonly delayed for 11 to 13 years, but it is also delayed in children.26,32 The clearest case in the present study was case 22, an adolescent whose mother explained that his gastrointestinal symptoms began when he was very young, but that his neurologic symptoms were more remarkable. For this reason the CD diagnosis was not made until he was 16 years old.
Of the 24 cases presented herein, biopsies of the intestinal mucosa were only taken in 6 of them. According to the ESPGHAN, if there is a strong clinical suspicion of CD and positive specific antibodies, HLA-DQ2 and DQ8 analysis can aid in ruling out CD diagnosis.1 Thus, in addition to the 6 patients with positive biopsy, only 2 more could be considered diagnosed. Nevertheless, 7 cases were of children under the age of 2 years, whose specific antibody titers might not be very high, as mentioned before. In 3 other cases, the titers were high but the haplotypes could not be analyzed at the time they were being seen. In the rest of the cases, biopsies were not taken because the parents would not allow it. However, especially in the children with typical CD, the symptoms unequivocally remitted with the gluten-free diet and the antibody indices became negative.
On the other hand, in addition to their genetic predisposition, the lactation and complementary feeding regimens were inadequate for the majority of the study patients. In this population only 29% of the children were breast-fed until 3 months of age, and cereals were introduced into their diet very early.33–35 According to Ivarsson et al.,36 this is a factor that promotes the development of CD in genetically predisposed children.
ConclusionsIn conclusion, the cases were grouped together by similarities in the symptomatology. Typical CD was the most common type in the youngest children; atypical CD, without malabsorption symptoms, was found in children of different ages; CD with gastrointestinal and extraintestinal (such as neurologic) symptoms; and gluten-related disorders with no CD. This manner of classification was used to describe the patterns presented by the cases in the study population.
CD should be evaluated in the context of clinical signs, serologic markers, haplotypes, and intestinal biopsy histology, along with the response to a gluten-free diet, and in different geographic regions, in order to have a complete description at the national level. The criteria of the ESPGHAN should be followed for making the definitive diagnosis of CD.
Financial disclosureThe study was partially funded by the S0008-2009-1/115212 Sectorial Salud project and the Conacyt (Mexico).
Conflict of interestThe authors declare that there is no conflict of interest.
The authors wish to thank the chemist JR Valenzuela Miranda for the technical assistance in the analysis of the specific antibodies, Melissa Ruiz Dyck M.S. for the haplotype analysis, and Adriana V. Bolaños M.S. for the final edition of the manuscript.
Please cite this article as: Sotelo Cruz N, Calderón de la Barca AM, Hurtado Valenzuela JG. Enfermedad celiaca en niños del noroeste de México: características clínicas de 24 casos. Revista de Gastroenterología de México. 2013;78:211–218.