¿ Introduction
Nearly 600 000 new cases of colorectal cancer (CRC) are diagnosed each year worldwide, representing 9% of cancer patients. In 2001, 3320 new cases of CRC were registered in Mexico, making it the second most frequent digestive tract cancer.1 CRC is the eighth cause of cancer-related death in women and the sixth in men.1 CRC is more frequent in people over 50 years of age and can be asymptomatic for long periods of time, only showing symptoms at advanced stages. The risk of CRC increases with age with only 3% of cases diagnosed in people under 40 year-old. The incidence is 19 and 337 per 100 000 in people under and over 65 years of age, respectively. An individual in a high incidence area has a lifetime risk of developing CRC of up to a 5%.1 Ninety percent of the time CRC arises from an adenoma; these premalignant lesions have prevalence between 10% and 35% in western societies. Up to 18% of people over 50 will develop adenomas. Adenoma´s resection has been shown to reduce the risk of developing CRC and mortality by up to 43%.2-5
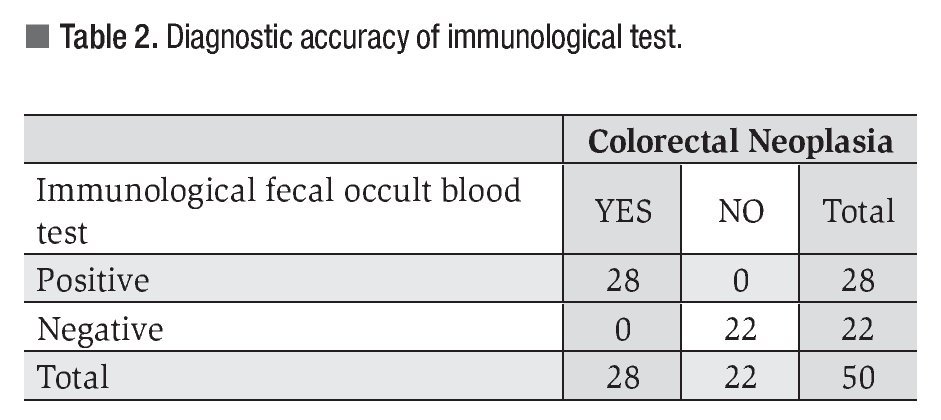
Biochemical and immunological fecal occult blood tests are based on the fact that almost 100% of advanced cancers and 60% to 80% of polyps bleed. These tests have a high positive predictive value for diagnosing gastrointestinal pathology in asymptomatic patients.2-4,6,7 Biochemical tests, such as Guaiac, have a sensitivity of 75% with a specificity of 34%, and a positive predictive value of 12%.6-11 Immunological tests have greater sensitivity (between 91% - 95%) and specificity (96% - 98%), and a positive predictive value of 60%. Besides the high sensitivity and specificity of immunological tests, these only react to human hemoglobin, differentiating them from guaiac tests, which react to any type of hemoglobin and to vegetable peroxidase. Immunological tests do not require any special diet prior to the test, are not altered by gastric or pancreatic digestive enzymes or by the metabolism of enteric bacteria.6-11
Also, iron deficiency anemia (microcytic and hypochromic (<80 μm3), is a well-known sign of advanced CRC.12-15 The World Health Organization defines anemia as a serum hemoglobin less than 13 g/dL.12-15 Serum ferritin, an acute phase reactant, is a water soluble trivalent iron compound bound to a protein, and in this way it is deposited in the liver, bone marrow, spleen, and other tissues. Ferritin depletes before hemoglobin, making it a useful marker for the selection of colonoscopy candidates.12,14-17
¿ Objective
Determine and compare the diagnostic value of immunological fecal occult blood test vs. serum ferritin levels for the detection of colorectal neoplasia (cancer or polyps) in high-risk patients.
¿ Methods
A prospective study was conducted at the National Cancer Institute (INCAN) in Mexico City between January 2007 and February 2008. A series of consecutive asymptomatic high-risk patients were selected and submitted to both immunological fecal occult blood test and serum ferritin; the results were compared against the standard test (colonoscopy plus histopathology).
Patients over 18 years of age, both genders at high risk for CRC (defined as family history of CRC, poliposis coli syndromes, or post-operative, diseases frees CRC) were considered for the study. Patients taking anticoagulants or non-steroidal anti-inflammatory drugs, previous or current radiation therapy, unable to undergo colonoscopy, chronic anemia of known cause (chronic renal insufficiency, rheumatic diseases, cancer, hemato-logical disorders), known causes of lower gastrointestinal bleeding (diverticular disease, ulcerative colitis, Crohn's disease) were excluded. Patients who did not complete diagnostic tests were eliminated from the study protocol.
The immunological test was performed before the colonoscopy. A kit (OCHemodia®) was used with a positive cutoff quantitative value of ≥50 ng/ mL or 6 μg/g hemoglobin. The stool sample was placed in a buffer solution and diluted to stabilize the hemoglobin, according to the manufacturer's instructions. Then a latex agglutination reaction was used to detect the presence of human hemoglobin antibodies.
Level of ferum ferritin (ng/mL) was determined before performing the colonoscopy.
All subjects were submitted to colonoscopy using a magnifying video colonoscope (Q160ZL, Olympus). The location and size of the lesions were recorded in photos. Biopsy specimens were taken of all identified lesions to determine the presence of colorectal neoplasia (polyps or CRC).
Statistical analysis: The sensitivity (Sn), specificity (Sp), positive predictive value (PPV), negative predictive value (NPV) were calculated with 95% confidence interval (CI95%) for both tests. Additionally ROC curve18 was calculated for the serum ferritin for the detection of CRC and precursor lesions at time. The categorical variables (IFOBT and/or colonic lesions positive or not) were analyzed using chi-square test with 1 degree of freedom and a significance level of 0.05 and the Fisher exact test. We used Student's t-test for quantitative variables (independent samples) and Variance analysis was performed with a significance level of 0.05 for variability between groups (age).
¿ Results
Fifty out of 54 patients were included in the study and 4 subjects were excluded. Twenty four were female and 26 males, mean age was 51 (range 19 - 88) and 44 (range 18 - 73), respectively. All patients were asymptomatic at the time of the study. Age and body mass index were not statistically significant, while the mean serum albumin level was significant lower in the patients with CRC (Table 1).
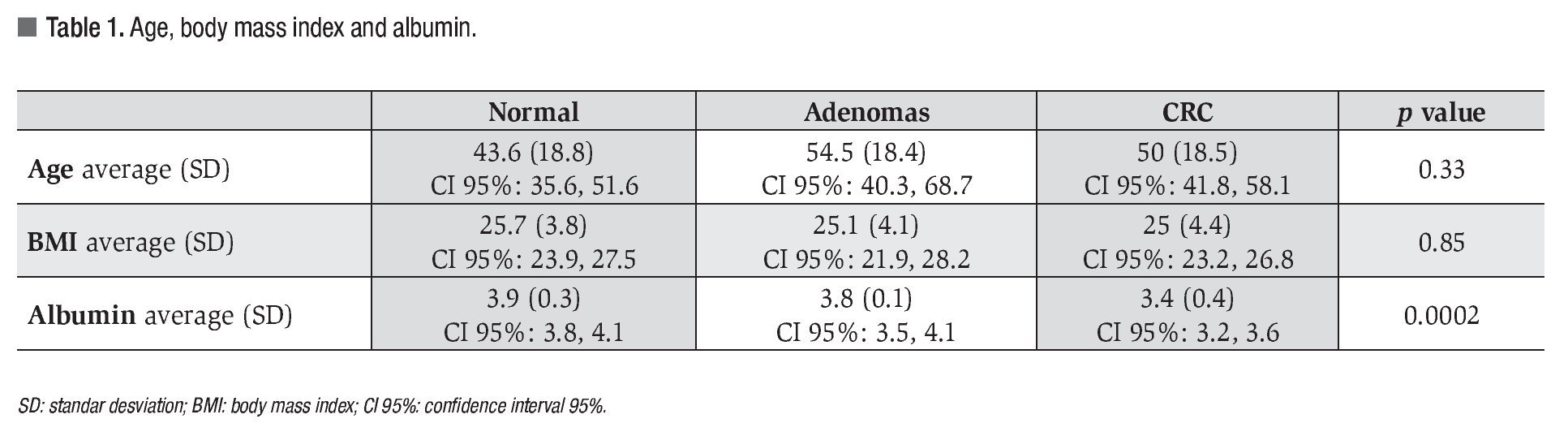
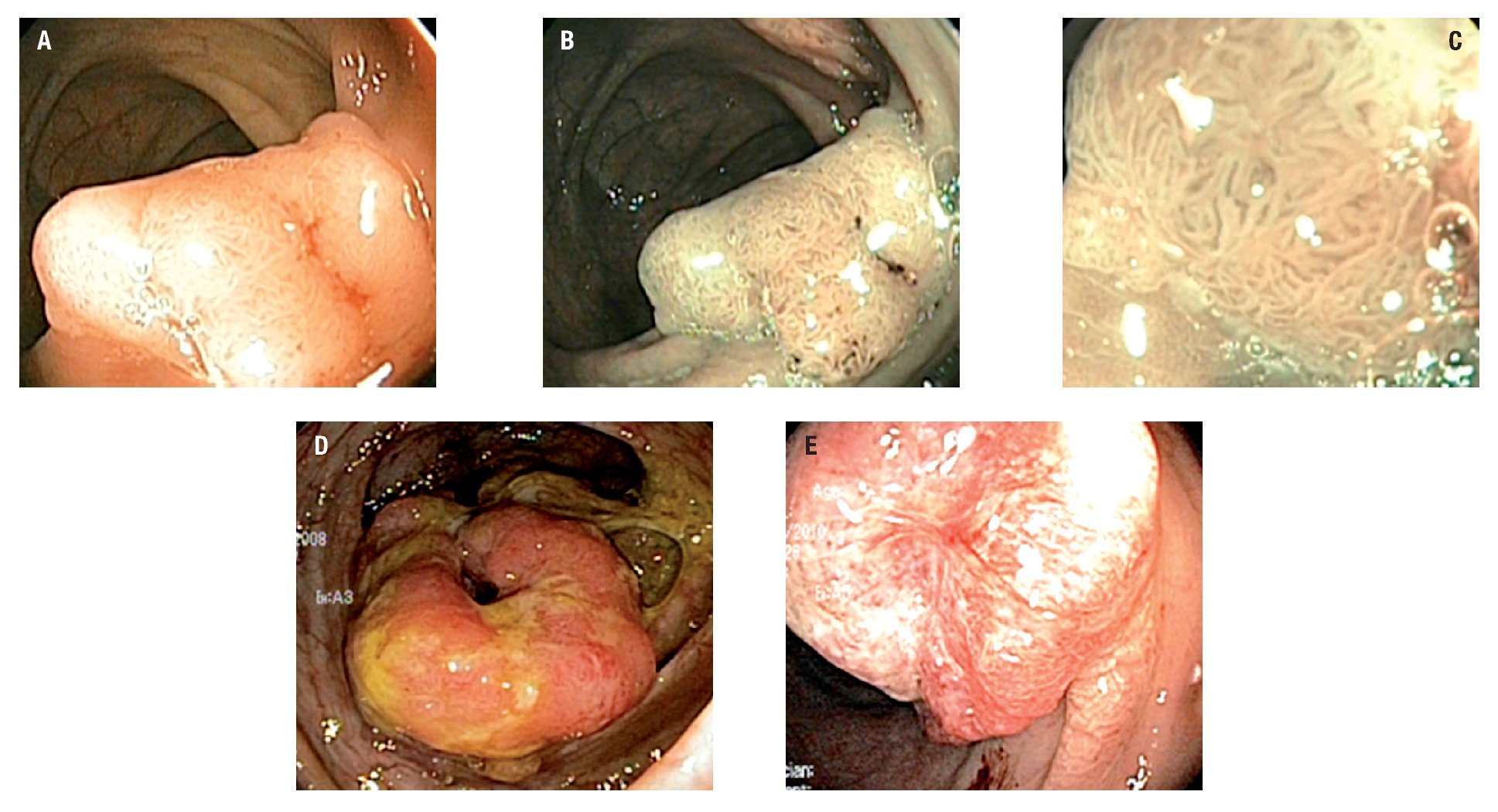
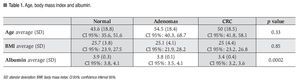
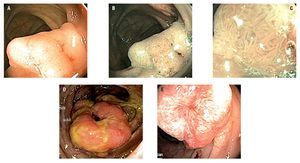
Twenty eight patients had colorectal neoplasia: 21 CRC and 7 adenomas. Between the patients with CRC, 16 were located in the sigmoid colon, 2 at the descending colon, 2 at the ascending colon and 1 in the cecum; all were larger than 5 cm. Five out of 7 adenomas found met criteria for advanced adenoma (high grade dysplasia or >10 mm or >25% villous component): 5 were villous adenomas (2 rectal, 2 in the ascending colon and 1 cecal) and 2 were tubular adenomas (1 rectal and 1 in the transverse colon) (Figures 1 and 2).
¿ Figure 1. Advanced adenoma (1A, 1B and 1C) and CRC (1D and 1E).
¿ Figure 2. Colorectal cancer.
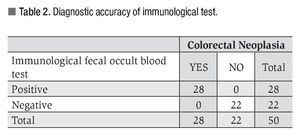
All patients with colorectal lesions tested positive at immunological test with a diagnostic sensitivity of 98% (Table 2).
The average result or serum ferritin in patients with CRC was 73.6 ng/mL (SD 74.2, CI 95%: 42.13, 105.3 and median 44); 60.1 ng/mL (SD 35.6, CI 95%: 5.507, 114.9 and median 49) in adenomas and 89.7 ng/mL (SD 77.2, CI 95%: 58.93, 120.6 and median 60.2) with negative colonoscopy (p = 0.58).
The average value of hemoglobin in patients with CRC was 12.9 g/dL (SD 1.6, CI 95%: 12.32, 13.66 and median 12.9), 13.3 g/dL in adenomas (SD 1.1, CI 95%: 12.14, 14.46 and median 14), and 14.6 g/dL with negative colonoscopy (SD 1.4, CI 95%: 14.03,15.35 and median 14). (p = 0.002).
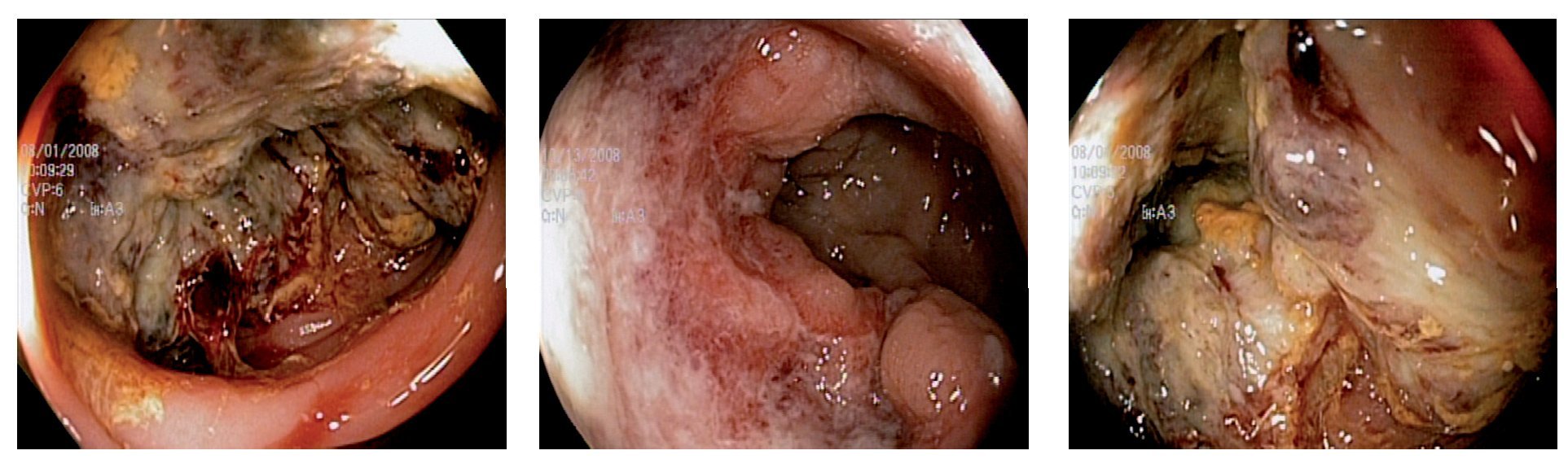
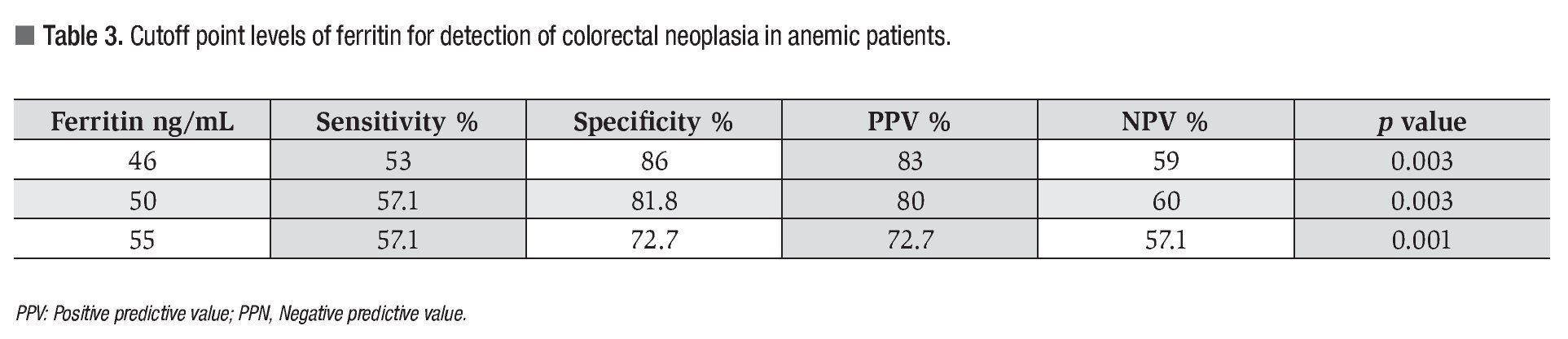
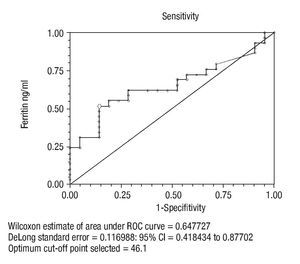
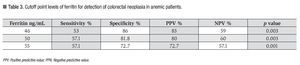
The cutoff point for ferritin was calculated using the ROC curve. It was determined that a serum ferritin level ≤46 ng/mL showed a significant correlation with colonic neoplasia (Figure 3). In anemic patients (Hb <13 g/dL) with a ferritin level <46 ng/mL, the test had a Sn of 53%, Sp 86%, PPV 83%, and NPV 59% (p = 0.003) (Table 3).
¿ Figure 3. Cutoff point of Ferritin in anemic patients.
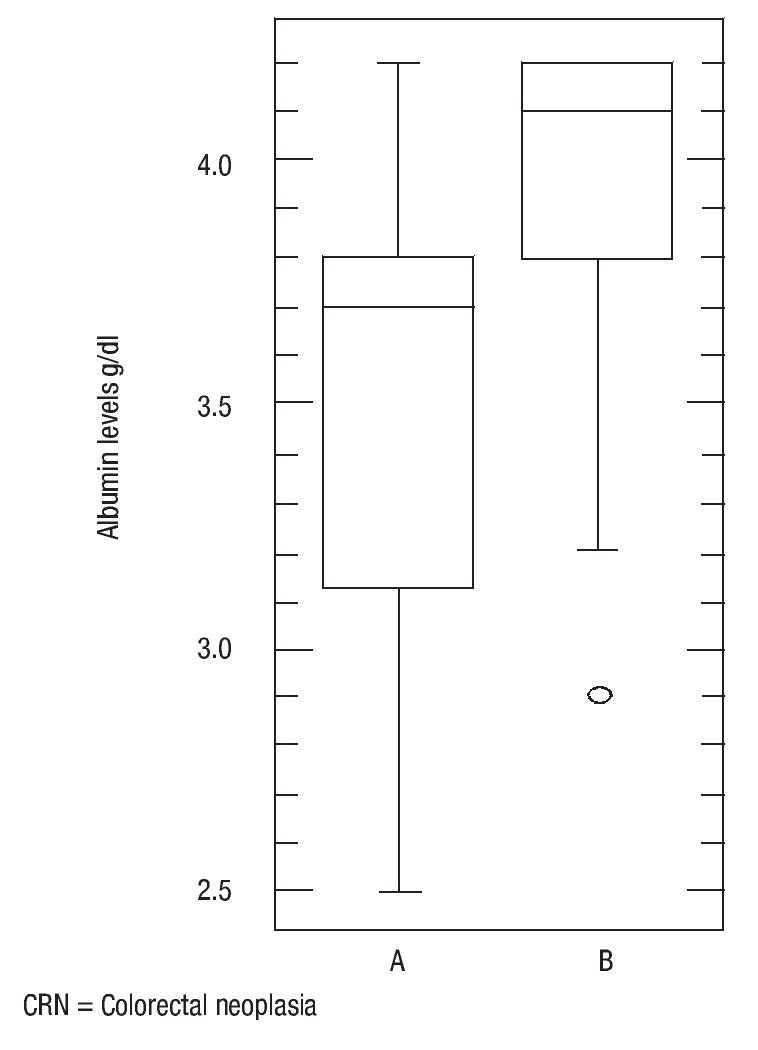
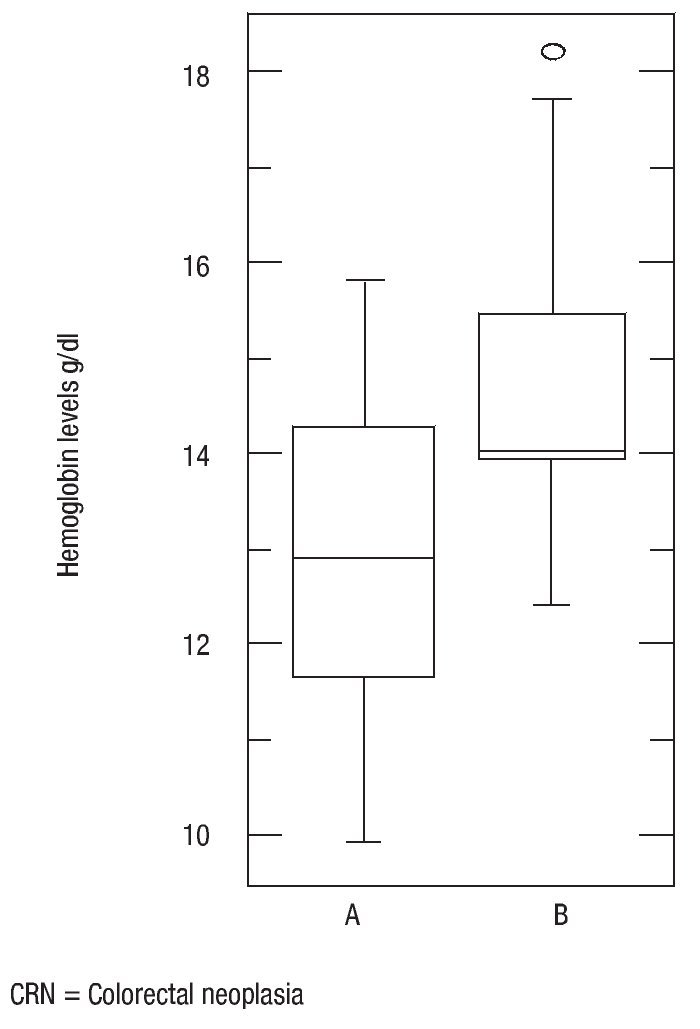
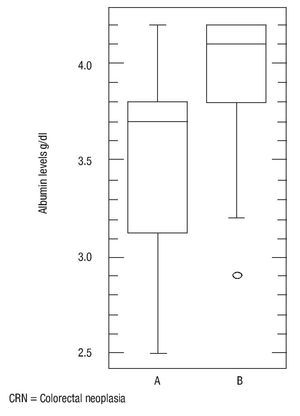
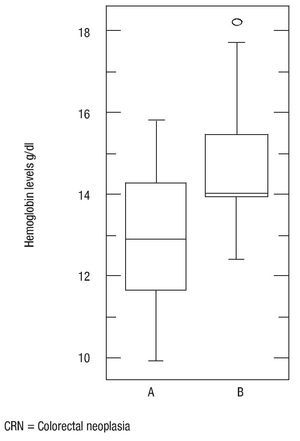
There was no statistically significant difference in age (p = 0.16), body mass index (p = 0.56) and ferritin levels (p = 0.34) between patients with colorectal neoplasia and healthy controls. However the difference in mean serum albumin levels (p = 0.0005, 3.5, CI95%: 3.392,3,701 vs. 3.9, CI 95%: 3.803,4,151) (Figure 4) and hemoglobin (p = 0.001, 13, CI 95%: 12.29,13,69 vs. 14.7, CI95%: 14.01,15,37) was statistically significant (Figure 5). The Sn, Sp, PPV and NPV for Hemoglobin only in anemic patients (<13 g/dL) were 46%, 91%, 87% and 57%, respectively.
¿ Figure 4. Albumin levels in patients with colorectal neoplasia and normal.
¿ Figure 5. Hemoglobin levels in patients with colorectal neoplasia and normal.
¿ Discussion
The quantitative immunological fecal occult blood tests are more sensitive and specific than biochemical tests for the detection of colorectal neoplasia (advanced adenoma and cancer)6-10 In countries like Uruguay, where the incidence of CRC is 39.6/100 000 people for men and 29.5 for women, with a mortality of 18.4 and 14.2/100 000 for men and women respectively, Fenocchi and collaborators6 performed a CRC screening study in average risk population using the immunological fecal occult blood test from OC Hemodia (Fecatest©). The overall analysis showed neoplastic lesions in 330 of the 11,734 patients submitted to Fecatest© (2.8%); of these 101 (30.6%) had cancer and 229 (69.4%) had high-risk adenomas with a detection rates of 0.86% and 1.95% respectively. The PPV for neoplasia (adenoma or cancer) was 37.5 (330/879 of IFOBT positive) and 11.5 (101/879 of IFOBT positive) for cancer.6
In our study we found the immunological test to be highly sensitive and specific for colonic hemorrhage secondary to CRC and/or adenomas, with sensitivity of 98%, higher than the ones reported in the literature (Sn 91% - 95% and Sp 96% - 98%).6-10 The sample included in this study is small compared to the aforementioned study,6 but was performed in high risk asymptomatic individuals.
In Australia, the incidence of CRC is 34.8/100 000 people with a mortality rate of 35 for men and 20.9 for women,19 Viiala and collaboratos8 studied the relationship between time of colonoscopy ad the diagnosis of CRC in 1632 average risk patients suspected of having CRC. In all patients over 65, a positive fecal occult blood test and iron deficiency anemia were positive predictive factors for CRC. The timing of colonoscopy was not significant.8 This supports the use of fecal occult blood tests in anemic patients to detect CRC. Based on this, we compared two tests, one for fecal occult blood and one for iron deficiency, finding that the first has a higher sensitivity and specificity for the detection of colorectal neoplasms.
Levi and collaboratos10 in Tel Aviv, Israel (incidence of CRC of 3.8 per 100 000 inhabitants, mortality rate: 3.5 for men and 2.5 for women),19 conducted a transversal study of 252 high risk patients for hereditary non-polyposis CRC using the immunological fecal occult blood test (OC-Micro 100 ng Hb/mL). Their results were as follows: Sn 100%, Sp 90%, PPV 16% and NPV 100% for cancer and Sn 74%, Sp 93%, PPV 45%, and NPV 98% for advanced adenomas. Seventy four percent of neoplasms were identified with a single test.10 We believe immunological tests can be used as a screening method to select the need for colonoscopy in high-risk populations and to minimize unnecessary tests. In another transversal study,11 the same authors compared the combination of the biochemical test (guaiac) plus the immunological test (OC-Micro) for fecal occult blood against the guaiac test alone. The number of colonoscopies needed-to-detect significant neoplasia with a positive guaiac was 6 to 8, while only 2 with the immunological test. The cost was 21% to 31% higher when using the guaiac test alone.11 We diagnosed colorectal neoplasia with a single colonoscopy after performing the immunological test in high-risk population, therefore the costs were lower than those reported by Levi.
In Minnesota USA, where CRC has an incidence of 32.8 per 100 000 inhabitants with a mortality rate of 35 for men and 20.9 for women,19 Mandeep and collaborators12 measured serum ferritin levels in anemic (Hb <13 g/dL) vs. non-anemic patients to determine the prevalence of CRC. They performed colonoscopies on 414 anemic and 323 non-anemic patients. The prevalence of CRC in anemic patients, with serum ferritin of 51-100 ng/mL or≤50 ng/mL was similar (7.9 and 7.2% respectively) while the incidence in non-anemic patients with ferritin between 51ng/mL - 100 ng/mL was 1.7% and 1.2% for those with ferritin levels ≤50 ng/mL. The Sp, PPV and NPV for CRC with ≤50 ng/mL ferritin were 77%, 41%, 8%, and 97% respectively and for a ferritin level of 51 ng/mL - 100 ng/mL were 92%, 28%, 8%, and 98% respectively. It was determined that a cutoff point of <100 ng/mL of serum ferritin can be used to determine which patients with anemia should undergo colonoscopy.12
Our results show that a cutoff point of serum ferritin set at 46 ng/mL for anemic patients (Hb <13 g/dl) has 53% sensitivity and 86% specificity for the detection of CRC. The differences in serum ferritin were not statistically significant between the groups. Nevertheless, anemia did make a difference between patients with and without neoplasia. Iron deficiency anemia is closely related to gastrointestinal malignancy as shown by Killip and collaborators.14 Of 700 adult patients with iron deficiency anemia, 6% were diagnosed with gastrointestinal cancer.14 Chronic anemia in high-risk patients must be studied, as this constitutes a risk factor for CRC by itself, and using serum ferritin we can select candidate patients for colonoscopy when levels are below 46 ng/mL.
Other iron deficiency markers have been studied. Beale20 examined the prevalence of iron deficiency amongst CRC patients. The transferrin saturation and serum ferritin levels were used as markers for iron deficiency. Their results suggest that transferrin saturation is more sensitive than serum ferritin for detecting iron deficiency.20
There are other screening tests such as fecal deoxyribonucleic acid (DNA) test. This is a relatively new technique that is more expensive and less sensitive than the immunological fecal occult blood tests.21 CT colonography is another alternative screening test. It can detect polyps 38 mm.22 The CRC screening guidelines currently include both biochemical and immunological tests to search for occult blood in the stool.2-4 The use of in-home biochemical fecal occult blood test has been successful in North America,23-25 however we recommend that due to the higher diagnostic accuracy of the immunological test, the biochemical test should be replaced by immunological determination of fecal occult blood for the screening of high-risk patients, therefore reducing the number of unnecessary screening colonoscopies performed.
¿Conclusion
The fecal occult blood immunological test is a better diagnostic tool than the determination of serum ferritin for the detection of CRC and adenomas. The hemoglobin level is more sensitive than serum ferritin for the detection of colorectal neoplasia in anemic patients.
Correspondence author: Dr. Sergio Sobrino-Cossío.
Av. San Fernando No. 22, Col. Sección XVI, Tlalpan C.P. 14080, México, D. F.
E-mail: ssobrinocossio@prodigy.net.mx
Received January 27th, 2011;
accepted April 8th, 2011.