¿ Introduction
Up to 3% of the world's population, or 170 million people, are chronically infected with hepatitis C virus (HCV).1 The prevalence of chronic HCV infection ranges from 0.1% to more than 10% in different countries, with the highest prevalence rates (10% to 15%) found in the African and Eastern Mediterranean regions. It is estimated that there are 2.7 to 3.9 million HCV chronic carriers in the United States and 5 million HCV carriers in Western Europe, with a higher prevalence of HCV in Eastern Europe.2-4 Recent reports indicate that HCV infection currently accounts for up to two-thirds of newly diagnosed cases of chronic liver disease in the United States.5
In Mexico, liver disease ranks fifth among most common causes of death, with alcoholic liver disease being the dominant etiology, followed by nonalcoholic fatty liver disease (NAFLD), and chronic hepatitis C.6 A systematic review of reactivity to antibody against HCV (anti-HCV) in Mexico showed a weighted mean prevalence rate of 0.37%, lower than prior estimates of 1%.7 HCV can be classified into six major genotypes based on sequence divergence of 30%, which differ by geographic distribution, with HCV genotype 1 being the most prevalent one in North America, South America, and Western Europe. Genotype 1 affects two-thirds (63-70%) of individuals with chronic hepatitis C in Mexico, similar to the prevalence of this genotype in the United States.7
HCV is efficiently transmitted by percutaneous exposure to infectious blood. Following acute HCV infection, up to 85% of adults will develop chronic infection and approximately 20% (range 5-25) of chronically infected individuals may develop cirrhosis after 25 to 30 years of infection.8 Children infected at a young age and women have lower rates of cirrhosis on long-term follow-up (2-6%). Persons most likely to have chronic HCV infection are those who received a blood transfusion prior to 1992 or have past or current injection drug use. However, other groups are at risk, such as those with human immunodeficiency virus (HIV) infection or history of multiple sexual contacts, persons with hemophilia who received clotting factor concentrates before 1987, patients who have been on hemodialysis, persons who received an organ transplant before 1992, those with unexplained elevation of serum aminotransferase levels, and children born to HCV-infected mothers. Chronic HCV infection is a major cause of morbidity and mortality, and may progress to cirrhosis, liver failure and hepatocellular carcinoma (HCC) with the need for liver transplantation, albeit at variable rates.9 Chronic hepatitis C is the leading indication for liver transplantation in many countries, accounting for more than one third (37-41%) of all liver transplants in the United States.10
Several factors appear to accelerate the progression of chronic hepatitis C, including advanced age, male gender, excessive alcohol intake, HIV coinfection, and hepatic steatosis with obesity, diabetes mellitus and insulin resistance.11,12 In addition, higher serum alanine aminotransferase (ALT) levels are associated with a higher rate of fibrosis progression, while worsening of fibrosis is less common in patients with persistently normal ALT levels.13 Chronic hepatitis C with advanced hepatitis fibrosis, and particularly with cirrhosis, is significantly associated with the development of HCC.14,15 Although chronic hepatitis B virus (HBV) infection is the most common predisposing factor for HCC worldwide, HCV infection is the most frequent cause of HCC in the United States and the incidence of HCV-associated HCC is increasing.14,15 Antiviral therapy may have a significant impact on the natural history of chronic HCV infection, as a sustained virologic response (SVR) to therapy may halt fibrosis progression, decrease the risk of HCC, and prolong survival.16-19
¿ Treatment
Guidelines for Treatment of Chronic Hepatitis C
The dominant current guideline for the treatment of chronic hepatitis C was developed by the American Association for the Study of Liver Diseases (AASLD) and published in 2009.20 Other guidelines on treatment of HCV infection are either outdated or focused on regional considerations of specific countries, such as different genotype prevalence rates, reimbursement policies, or other local issues. Although a bit dated, the position statement from the American Gastroenterological Association (AGA) published in 2006 is also frequently cited along with the AASLD guideline.21 The earlier hepatitis C consensus development conferences conducted by the National Institutes of Health (NIH) in 1997 and 2002 were historically instrumental in providing guidance and standardization to the management of patients with chronic hepatitis C.22,23 In the recent past, a joint effort of the AGA and the American Medical Association led to the development of hepatitis C Physician Performance Measures, which have been adopted by the United States Centers for Medicare & Medicaid Services as part of the Physician Quality Reporting Initiative, to assist physicians in enhancing quality of care in patients with chronic hepatitis C.24 These comprehensive measures focus on diagnostic testing, counseling and education, treatment of hepatitis C, and both hepatitis A and B vaccination. Finally, the Institute of Medicine just recently assembled an expert committee and published a report calling for improved surveillance for HCV and HBV, advances in knowledge and awareness of viral hepatitis, improved HBV vaccine coverage, and better integration of viral hepatitis services in the United States.25,26
Selection of Patients for Treatment
The diagnosis of chronic hepatitis C is made by the detection of anti-HCV and serum HCV RNA, which are usually obtained based on the presence of risk factors for HCV infection. 20,21 The role of liver biopsy as part of the baseline evaluation remains controversial, but biopsy is generally recommended for staging hepatic fibrosis in patients with genotype 1 infection to assist the patient and physician in making a decision regarding initiation of antiviral therapy.20 A liver biopsy may be unnecessary in patients with genotype 2 or 3 infection, since approximately 80% achieve an SVR. Although currently available noninvasive tests may be useful in defining the presence or absence of minimal or advanced fibrosis, the AASLD does not recommend these tests to replace liver biopsy in routine clinical practice.20
Current recommendations for the treatment of patients with chronic hepatitis C are primarily derived from the outcomes of randomized controlled trials (efficacy) involving patients selected based on restrictive inclusion criteria.20,21 However, it must be kept in mind that the results of therapy in clinical practice (effectiveness) may not be equivalent, as was shown in a New York study of 255 patients that included many ethnic minorities (58% Hispanic and 20% African Americans) who were naïve to prior therapy and had quite low SVR rates with standard peginterferon plus ribavirin therapy (14% in genotype 1 patients, and 37% in genotypes 2 and 3 patients).27 Standard indications, relative indications, and contraindications for treatment of chronic hepatitis C recommended in the AASLD guideline are summarized in Table 1. Treatment indications for patients in real-life settings are more challenging, and the risk and benefits of therapy need to be weighed in these circumstances, such as in patients with psychiatric disorders, advanced or young age, various medical comorbidities, history of poor adherence, and other psychosocial or medical issues. Chronic hepatitis C disproportionately affects minorities and lower socioeconomic groups, who often are not candidates for therapy and have lower SVR rates when treated.27 Despite the substantial improvements in therapy for chronic hepatitis C, the rates of diagnosis and treatment are low, and treatment rates appear to be declining in the United States.28 One analysis of ambulatory care visits showed that less than 10% of visits were associated with a prescription for antiviral therapy, regardless of demographic and insurance status.29 Another study of patients receiving care through the Veterans Administration, which covers a population with a known high rate of HCV infection, showed that only 12% of veterans diagnosed with chronic hepatitis C received a prescription for antiviral therapy.30 Efforts to improve rates of diagnosis (less than half of all HCV-infected individuals) and treatment uptake are required to ameliorate the future health care burden of hepatitis C from the development of cirrhosis and HCC.
Current Standard Treatment
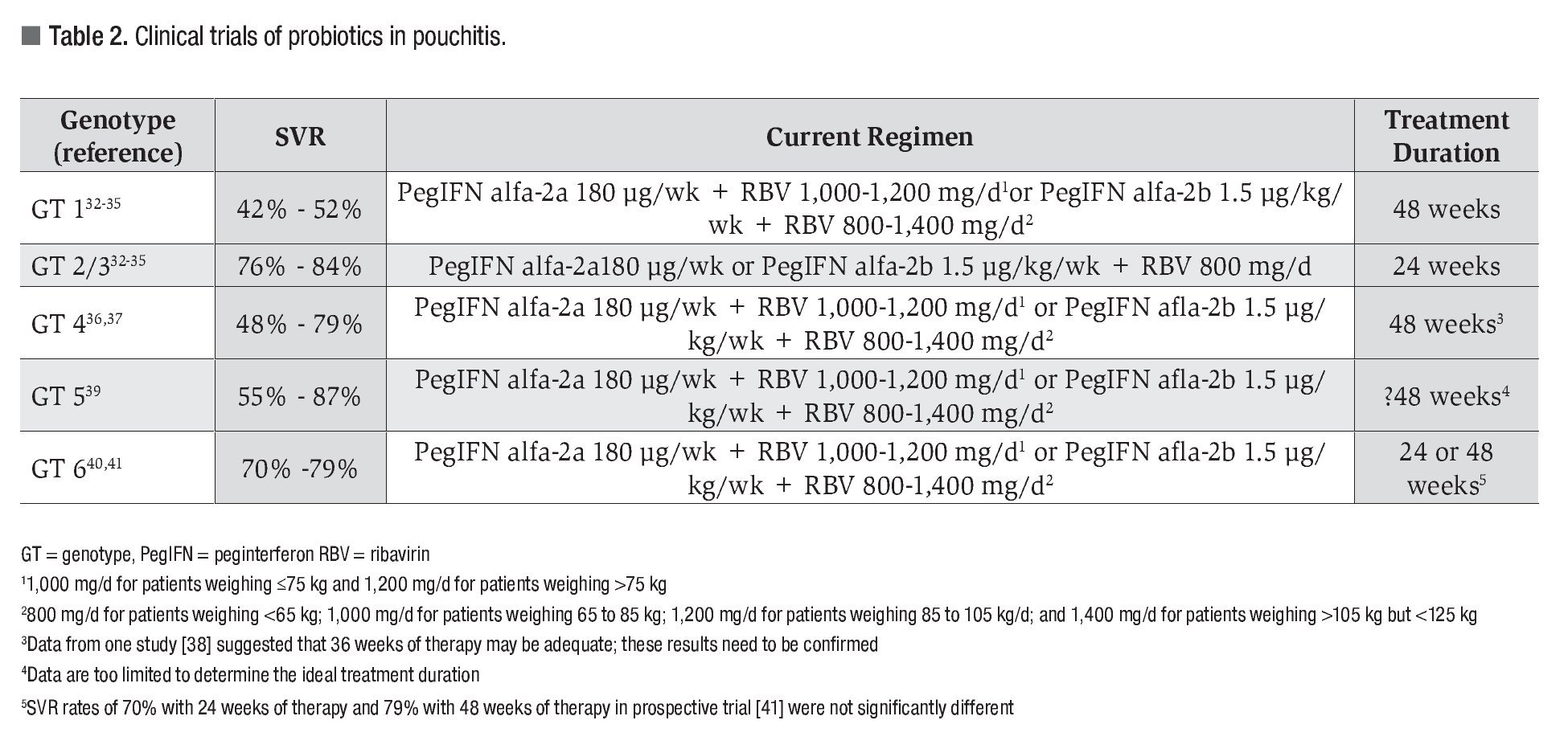
There has been impressive progress in the treatment of chronic hepatitis C since the first use of standard interferon monotherapy in the early 1990s, which overall achieved SVR rates of approximately 10%.31 With the addition of ribavirin in the late 1990s followed later by pegylation of interferon to enhance its half-life, the SVR rate increased to approximately 50%-60% overall using peginterferon and ribavirin based on the results of three pivotal trials.32-34 These studies showed that SVR rates ranged from 42% to 52% in patients with genotype 1 infection treated with peginterferon alfa-2a (Pegasys®, Roche Pharmaceuticals Inc., Nutley, NJ, USA) or peginterferon alfa-2b (Peg-Intron®, Schering-Plough Corporation, Kenilworth, NJ, USA) plus ribavirin for 48 weeks, and 76% to 84% in those with genotype 2 or 3 infection treated for 24 or 48 weeks (Table 2). The study by Hadziyannis et al.34 demonstrated that 24 weeks of combination therapy using ribavirin at a dose of 800 mg daily was adequate for patients with genotype 2 or 3 infection. The initial dose of ribavirin used in the interferon alfa-2b registration trial was 800 mg daily,32 but a subsequent large community-based trial demonstrated that weight-based ribavirin (800-1400 mg daily) was superior to flat-dose ribavirin (800 mg/d) when used in combination with peginterferon alfa-2b for the treatment of patients with genotype 1 infection.35 Thus, combination therapy with either peginterferon alfa-2a or peginterferon alfa-2b and weight-based ribavirin (1000-1200 mg with peginterferon alfa-2a, and 800-1400 mg with peginterferon alfa-2b) for 48 weeks is the current standard of care for the treatment of genotype 1 HCV infection, and either of the peginterferon products plus ribavirin 800 mg daily for 24 weeks is used for patients with genotype 2 or 3 HCV infection.20,21
There are fewer studies and less data regarding the outcomes of therapy for patients with genotype 4, 5 or 6 infection. Patients with chronic hepatitis C genotype 4 infection appear to achieve maximal benefit with 48 weeks of peginterferon and weight-based ribavirin therapy, with SVR rates ranging from 48% to 79%,36,37 although limited data from one study suggests that 36 weeks of therapy is sufficient provided an early virologic response (EVR; a 32 log10 decrease in HCV RNA levels from baseline) is achieved (Table 2).38 There are no prospective studies on the treatment of patients with chronic hepatitis C genotype 5 infection, but five non-randomized retrospective studies using different interferon-based treatment regimens and durations of therapy demonstrated SVR rates varying between 55% and 87%.39 In HCV genotype 6 infection, which is common in Vietnamese patients, a small retrospective analysis of different treatment regimens found that higher SVR rates were seen in patients receiving 48 weeks of peginterferon plus ribavirin versus those treated for 24 weeks (75% vs. 39%).40 However, a subsequent prospective study did not show a significant difference in the SVR rates of patients treated for 24 or 48 weeks, i.e., 70% vs. 79% (p = 0.45).41
Peginterferon alfa-2a and peginterferon alfa-2b differ in their pharmacokinetics and dosing regimens.42 Peginterferon alfa-2b has a linear 12-kd polyethylene glycol (PEG) chain covalently linked to standard interferon alfa-2b via an unstable urethane bond that is hydrolyzed when injected. Peginterferon alfa-2a has a 40-kd branched PEG chain covalently linked via a stable amide bond to standard interferon alfa-2a and circulates as an intact molecule. Thus, peginterferon alfa-2a has a restricted volume of distribution, longer half-life and reduced clearance, and can be administered once weekly irrespective of body weight. Peginterferon alfa-2b has a shorter half-life, wide volume of distribution, and requires body weight-based dosing. Trials comparing the two peginterferons have had variable results although some randomized and retrospective studies appear to show superiority of peginterferon alfa-2a.42 The largest head-to-head randomized controlled trial conducted in the United States involving 3 070 patients with genotype 1 infection showed that peginterferon alfa-2a and peginterferon alfa-2b in combination with ribavirin had comparable efficacy in the treatment of chronic hepatitis C, with SVR rates of 40% in patients treated with peginterferon alfa-2b plus ribavirin 800-1400 mg daily and 41% in those treated with peginterferon alfa-2a plus ribavirin 1000-1200 mg daily.43 However, a 2010 meta-analysis including only head-to-head randomized controlled trials showed a significant advantage for peginterferon alfa-2a over peginterferon alfa-2b, with SVR rates of 47% vs. 41%.44 When patients with genotypes 1 and 4 or genotypes 2 and 3 were analyzed separately, the results continued to favor peginterferon alfa-2a. There were no differences with regard to adverse effects between the two drugs in this meta-analysis.
The goal of treatment of chronic hepatitis C is to prevent complications and death from HCV infection. Emerging data demonstrates that interferon-based therapy, particularly among those achieving an SVR,18,19,45 is associated with long-term persistence of SVR, improved fibrosis and inflammation scores, reduced incidence of HCC, improved quality of life, and prolonged life expectancy.16-19,46-49 This reduction in the rate of progression has also been demonstrated in patients with chronic hepatitis C and cirrhosis in some but not all studies. The impact on slowing progression is greatest in patients with an SVR, less in relapsers, and equivocal in nonresponders. Thus the natural history of chronic hepatitis C after completion of antiviral therapy is favorable with achievement of an SVR.
Viral Kinetics and Predictors of Virological Response
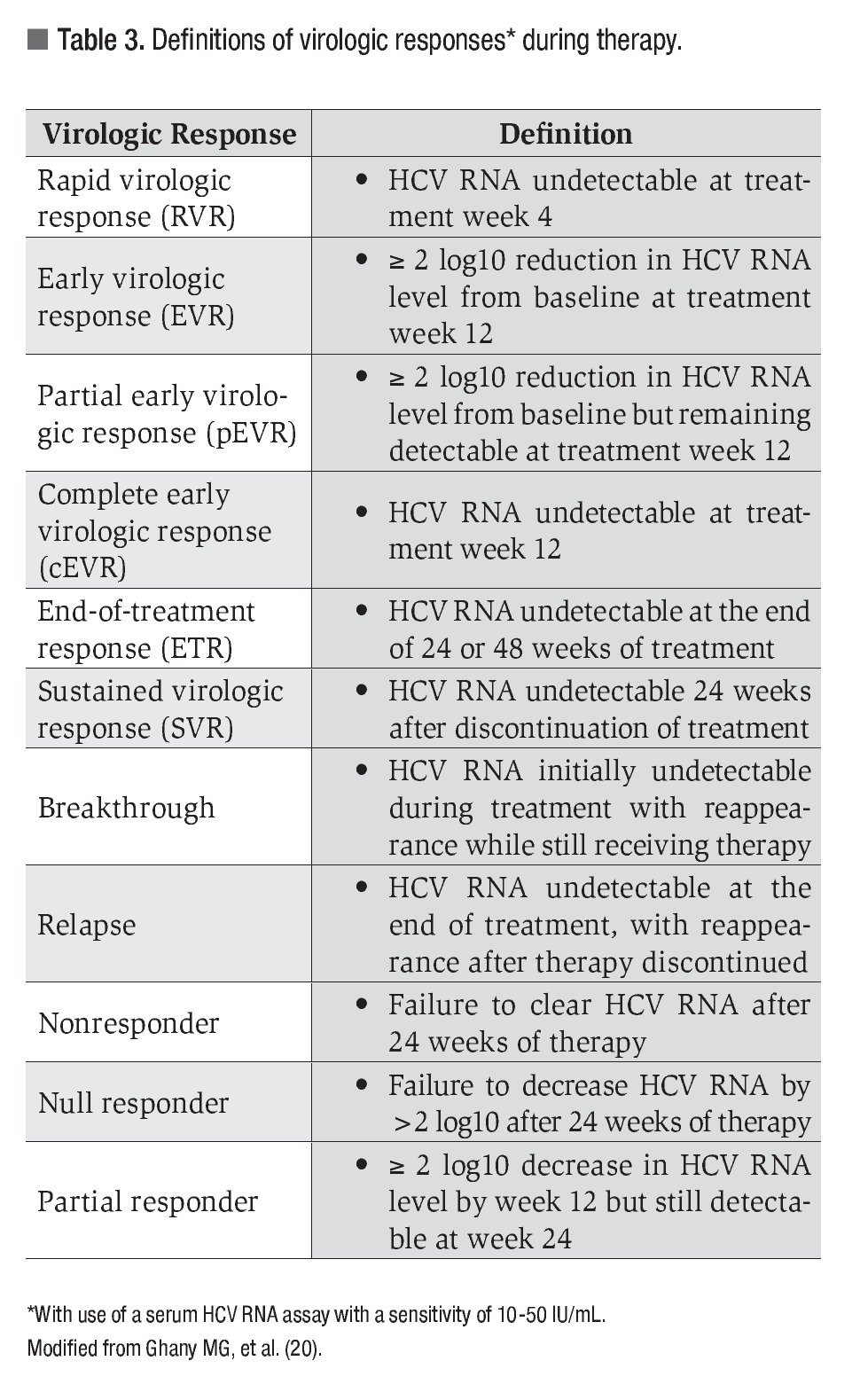
The timing of viral clearance of HCV from serum during therapy has been shown to be helpful as an early predictor of the likelihood of responding to therapy, for determining the optimal duration of therapy, and as a practical stopping rule in nonres-ponders. The common virologic responses during therapy and their definitions are shown in Table 3. The most important response is the SVR, which is generally regarded as a "virologic cure," although patients with cirrhosis remain at risk for HCC and require continued surveillance.20,21 The ability to achieve complete HCV RNA suppression to undetectable levels at four weeks of therapy, known as a rapid virologic response (RVR), is highly predictive of an SVR independent of genotype and has a positive predictive value for SVR in genotype 1 patients of up to 90%.50 However, only 15% to 20% of genotype 1 patients treated with standard therapy achieve an RVR. The achievement of an EVR, defined by at least a 2 log10 decrease in HCV RNA from baseline levels at week 12 of therapy, is also an important predictor of response to antiviral therapy. Patients who fail to achieve an EVR have a negative predictive value of SVR approaching 100% and are considered nonresponders in whom therapy should be stopped.33,51 A retrospective study of genotype 1 patients enrolled in six different trials of peginterferon plus ribavirin combination therapy provided evidence that complete suppression of HCV to undetectable levels at different time points is the most important on-treatment predictor of SVR.52 In this study, patients with an RVR had the highest rate of SVR at 87%. Patients who achieved a complete EVR (cEVR) had a significantly greater SVR rate (68%) compared with those who had a partial EVR (pEVR) with a 2 log10 reduction but still detectable serum HCV RNA at week 12 (SVR rate of 27%). Patients who did not achieve an EVR had the lowest SVR rate at 5%. Thus, more rapid complete clearance of HCV RNA is associated with a higher likelihood of achieving an SVR.
Several clinical and virological factors predict the likelihood of achieving an SVR to combination peginterferon and ribavirin therapy and may be used to guide management decisions both before and during a course of therapy.20,21 HCV genotype is an important predictor of response to therapy, with the highest SVR rates in patients with geno-type 2 infection, intermediate rates in genotypes 3, 4 and 6, and the lowest rate in genotype 1 infection (Table 2). A number of factors have been established as predictors of a reduced SVR rate, including high baseline serum HCV RNA levels (typically > 800,000 IU/mL), African-American or Latino ethnicity, increased age (particularly > 60 years of age), obesity, and the presence of advanced fibrosis or cirrhosis.20,21,53,54
A new predictor of response to therapy, which was uncovered by three independent genome-wide association studies, is a recently identified genetic variation in the IL28B gene that encodes interferon lambda-3 on chromosome 19.55-57 The CC genotype at the rs12979860 single nucleotide polymorphism in the IL28B locus demonstrates an extraordinarily strong relationship with the outcome of antiviral therapy of chronic hepatitis C genotype 1 infection. In the study by Ge et al.,55 the favorable CC genotype (compared with the CT or TT genotypes) was associated with a twofold increase in SVR rate in all ethnic groups and was a stronger independent predictor of SVR than viral load, fibrosis stage, or ethnicity. The CC genotype was more common in Caucasians than African Americans and was estimated to account for about half of the known discrepancy in response rates in these two populations.55 In addition, the CC genotype is most common in Asian patients, and thus this genetic variant may explain the higher response rates found in this ethnic group.58 It is expected that a licensed IL28B assay will be available soon and used in routine practice to determine the best course of treatment, such as a standard or a shortened course of therapy for those with the favorable CC genotype versus a more aggressive course of therapy with the addition of newer direct acting antiviral (DAA) agents in those with the unfavorable CT or TT genotypes.59
Evolving Strategy of Individualized Therapy
Patients who demonstrate rapid clearance of serum HCV RNA during treatment may be considered for discontinuation at an earlier time point, especially those with poor tolerance to treatment. In patients with genotypes 2 or 3 who achieve an RVR, a shortened course of therapy of 12 to 16 weeks has been proposed, particularly for patients with low baseline serum HCV RNA levels, normal platelet count, absence of advanced hepatic fibrosis, and a low body mass index (BMI); however, prospective studies have had critical differences in study design that may account for the mixed results that have been reported.60-66 The largest study by Shiffman et al.66 involving 1 469 patients who received peginterferon alfa-2a plus ribavirin 800 mg daily showed that the SVR rate was significantly lower in patients treated for 16 weeks compared to 24 weeks (62% vs. 70%). In addition, the relapse rate was significantly higher in the 16-week group (31% vs. 18%). Even among patients with an RVR, the SVR rates were significantly lower in the 16-week group (79% vs. 85%). However, SVR rates were similar in a subset analysis of patients with baseline serum HCV RNA levels £ 400,000 IU/mL (82% vs. 81%).66 A subsequent analysis of this trial restricted to patients who achieved an RVR and received treatment for 80% or more of the planned treatment duration once again showed a significantly higher SVR rate in patients randomized to 24 weeks of therapy (91% vs. 82%; p = 0.0006).67 SVR rates in patients with a baseline serum HCV RNA levels £ 400,000 IU/mL randomized to 24 and 16 weeks of therapy were similar (95% and 91%). Significant pretreatment predictors of SVR were assignment to 24 weeks of treatment, absence of advanced fibrosis on liver biopsy, lower HCV RNA levels, and a lower body weight.67 The seven trials studying a shortened course of therapy for patients with genotype 2 or 3 infection provide evidence for the following general conclusions: (1) patients with genotype 2 or 3 who achieve an RVR, particularly those with a low baseline viral load and without bridging fibrosis or cirrhosis, can be treated for less than 24 weeks, although the exact duration (12, 14 or 16 weeks) remains debatable; (2) patients with genotype 3 and a high viral load (> 800 000 IU/mL) are the most difficult to treat and need therapy for 24 and possibly even 48 weeks; and (3) patients with genotypes 2 or 3 without an RVR are not candidates for a shorter treatment duration, as even 24 weeks yields unsatisfactory SVR rates of 50-70%.67,68 Although the evolving literature makes a case for shortening therapy in selected patients with genotype 2 or 3 infection, the AASLD guideline recommends a standard 24-week course of therapy for patients with these genotypes, unless patients are intolerant of a planned 24-week course of therapy and understand that the relapse rate is higher with the shortened course of therapy.20
In patients with genotype 1 infection, dose modifications and variations in treatment duration are two strategies that have been investigated to optimize the outcomes of therapy.69 Studies have suggested that a shortened 24-week course of therapy in genotype 1 patients who achieve an RVR may be as effective as 48 weeks, particularly in those with low baseline HCV RNA levels, although the viral load cutoff has varied in different studies (400 000 IU/mL, 600 000 IU/mL, and 800 000 IU/mL). 70-72 A recent meta-analysis of seven studies found that patients with genotype 1 infection treated for 24 versus 48 weeks had a significantly lower SVR rate (-14%), which was related to a 10% higher relapse rate.73 However, treatment for 24 weeks in patients with a baseline serum HCV RNA level of £400 000 IU/mL was as effective as a 48-week course of therapy. The selection of patients with genotype 1 infection for a shorter course of therapy remains problematic, as the presence of factors other than viral load such as obesity, insulin resistance, and advanced fibrosis, among others, need to be considered. Thus, the AASLD and another expert panel do not generally recommended a shortened course of therapy for patients with genotype 1 infection, although the AASLD accepts that selected patients with an RVR and baseline HCV RNA £400 000 IU/ mL can be treated with a 24-week course of therapy with SVR rates approaching 90%.20,74
On the other hand, persistently detectable HCV RNA during therapy of genotype 1 infection predicts a low likelihood of achieving an SVR.
Patients who continue to have detectable HCV RNA at 24 weeks of therapy are nonresponders, have a very low likelihood of SVR, and should have therapy discontinued.50 In patients with detectable HCV RNA at week 12 who subsequently clear HCV RNA by week 24, often characterized as "slow responders," extending therapy to 72 weeks may have a favorable impact on the SVR rate.75-77 Although these three studies included patients that were not homogeneous, had different baseline characteristics, and used different treatment regimens, results showed trends toward higher SVR rates by extending therapy from 48 to 72 weeks that was primarily related to lower relapse rates. With 48 versus 72 weeks of therapy, the SVR rates were 32% vs. 45% in the study of Sanchez-Tapias et al.,75 17% vs. 29% in the study of Berg et al.,76 and 18% vs. 38% in the study of Pearlman et al.77 In contrast to these three studies, a recent prospective study did not demonstrate a significant advantage of extended treatment.78 The AASLD guideline recommends that consideration should be given to extending therapy to 72 weeks in patients with delayed viral clearance.20
Optimizing Adherence to Therapy
Peginterferon and ribavirin both have side effects that can lead to adverse events, dose reduction, and treatment discontinuation, and thus efforts to optimize adherence to therapy is essential to achieving an SVR. Peginterferon therapy can be associated with fatigue, flu-like symptoms, depression, anxiety, exacerbation of both psychiatric and autoimmune diseases, as well as hematologic side effects such as neutropenia and thrombocytopenia. One of the most important side effects associated with ribavirin therapy is hemolytic anemia, which must be monitored closely as it can be severe, particularly in difficult patient groups such as cirrhotics. If hematologic side effects occur, dose reductions may be required;20,21 however, growth factors may be used in selected cases.74 Efforts should be made to optimize ribavirin dosing, as it is a key factor in the prevention of relapse, and recent data suggest that reductions in ribavirin dosage that decrease the cumulative dose to below 60% may contribute to a poor outcome.79 In addition, adherence to peginterferon dosing has a significant impact on ability to achieve SVR based on a retrospective analysis that shows reduction of either interferon or peginterferon and/or ribavirin below 80% of the originally prescribed dose or stopping the medications before the patient received 80% of the planned duration were associated with a significant decrease in SVR.80 The largest reduction in SVR occurred in patients in whom dose was reduced or treatment was discontinued before 12 weeks of therapy; their SVR rate was only 34%.
Retreatment of Relapsers and Nonresponders
Approximately half of treated patients with chronic hepatitis C will fail standard therapy and may be considered as potential candidates for retreatment. Nonresponders are defined as patients with detectable serum HCV RNA after 24 weeks of therapy, relapsers as patients with undetectable serum HCV RNA at the end of treatment but detectable at 24 weeks of follow-up, and breakthrough patients as those who transiently have undetectable serum HCV RNA levels during therapy that subsequently become detectable during continued therapy (Table 3). Patients who have failed prior standard interferon therapy may be considered for retreatment with peginterferon plus ribavirin combination therapy. Three prospective studies have reported SVR rates ranging from 20% to 40% with peginterferon alfa-2a or alfa-2b treatment of nonresponders to interferon monotherapy and 8-10% in nonresponders to prior interferon and ribavirin therapy.81-83 Up to 58% of relapsers following treatment with standard interferon may achieve SVR with peginterferon plus ribavirin.82,84-89 Patients most likely to achieve SVR with retreatment include those with non-1 genotype, low baseline HCV RNA levels, a low fibrosis stage, and Caucasian race.
Patients most commonly seen today are those who have failed treatment with peginterferon plus ribavirin. In the Evaluation of Peg-Intron in Control of Hepatitis C Cirrhosis (EPIC3) study, patients who relapsed or were nonresponders to prior inter-feron (n=1203) or peginterferon (n=820) with or without ribavirin were treated with peginterferon alfa-2b and weight-based ribavirin.90 The SVR rate was 22% overall and 15% in patients with geno-type 1 infection, 59% with genotype 2 infection, and 45% with genotype 3 infection. However, SVR rates were less than 10% (6-7%) in nonresponders to prior peginterferon plus ribavirin therapy.90 This low response rate was confirmed in the REtreatment with PEgasys in pATients not responding to prior peginterferon alfa-2b (12kDa)/ribavirin combination therapy (REPEAT) trial (n=950), which showed an SVR rate of only 9% (all geno-types) with retreatment with peginterferon alfa-2a plus ribavirin.91 A higher induction dose of peginterferon alfa-2a (360 mg weekly for 12 weeks) did not increase the SVR rate when compared to the standard dose of 180 mg weekly, but extension of retreatment to 72 weeks was associated with a higher SVR rate (16% with 72 weeks vs. 8% for 48 weeks). However, based on these modest efficacy rates, the AASLD guideline does not recommend retreatment of patients who did not achieve an SVR with peginterferon plus ribavirin with another course of peginterferon plus ribavirin.20
Other formulations of interferon-based therapy have been introduced as alternatives for prior treatment failures, such as interferon alfacon-1 (consensus interferon). Interferon alfacon-1 is a synthetic interferon protein derived from a consensus sequence of the most common amino acids found in naturally occurring alpha interferon sub-types. Interferon alfacon-1 is administered daily when used for retreatment, which is a potential disadvantage of the use of this agent. Rates of SVR following therapy with interferon alfacon-1 in prior interferon monotherapy nonresponders and interferon plus ribavirin relapsers appear to be comparable to retreatment with peginterferon and ribavirin; however, several prospective studies have reported SVR rates ranging from 22% to 36% in prior interferon plus ribavirin nonres-ponders, which are higher than those reported following retreatment with peginterferon plus ribavirin.92 However, a large multicenter prospective, randomized, phase 3 trial has recently reported a modest SVR rate of 10% using interferon alfacon-1 15 mg daily plus weight-based ribavirin in nonresponders to peginterferon plus ribavirin.93 A subanalysis showed that prior null responders had a significantly lower SVR rate, and noncirrhotic patients with an EVR during prior therapy had a higher SVR rate of 29%.
In chronic patients who failed prior interferon-based therapy, the concept of maintenance therapy with low-dose peginterferon was introduced with the goal of delaying disease progression. Three large prospective studies evaluating this treatment strategy have been performed and have thus far not demonstrated any survival benefit.94-96 The Hepatitis C Antiviral Long-term Treatment against Cirrhosis (HALT-C) trial showed no difference in survival, incidence of HCC, clinical decompensation, or development of cirrhosis between treated and untreated patients over a 3.5 year follow-up in patients treated with peginterferon alfa-2a 90 mg weekly.94 Likewise, the Colchicine versus Peg-Intron Long-term (COPILOT) trial demonstrated no survival benefit to low-dose peginterferon alfa-2b 0.5 mg/kg/week monotherapy versus colchicine over a 4 year period; however, data from this study suggested that patients with portal hypertension treated with peginterferon had a lower incidence of variceal bleeding during the follow-up.95 Finally, in the EPIC3 trial, patients who did not respond to retreatment received peginterferon alfa-2b 0.5 mg/ kg/week monotherapy or no treatment for 3 (those with stage 2 or 3 fibrosis) or 5 (those with stage 4 fibrosis) years.96 Preliminary data indicate that maintenance therapy was not superior to control in the prevention of clinical events. The results of these three maintenance trials do not support long-term low-dose peginterferon in the management of patients with chronic hepatitis C and advanced fibrosis.20
Treatment of Special Groups
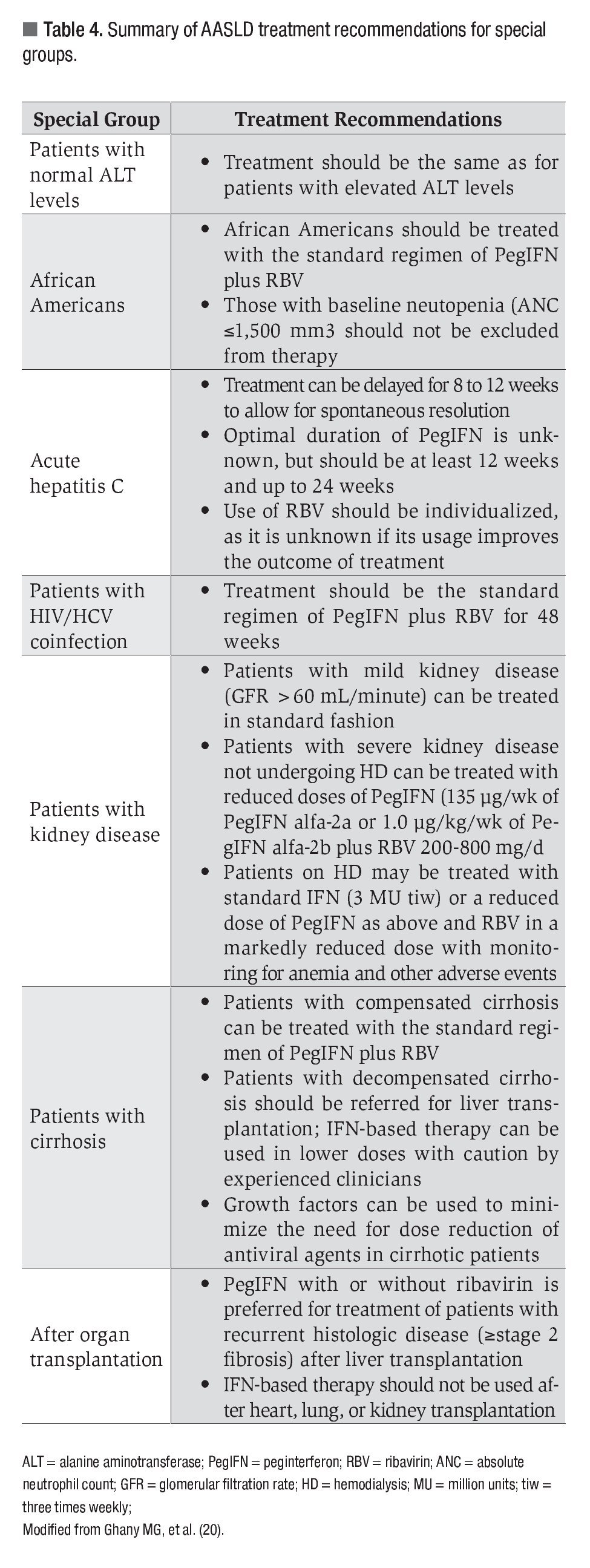
This concise review does not allow a detailed discussion of antiviral therapy of a number of special groups with chronic HCV infection. A summary of the recommendations of the AASLD for several of these patient groups may be found in Table 4.
¿ Future Treatments for Hepatitis C
Several new investigational antiviral therapies that selectively target various components of the HCV life cycle have now completed early-phase clinical trials and additional agents are currently in development. These agents include protease inhibitors, polymerase inhibitors, immune modulators, and other molecules. The DAA drugs that target the NS3/4A serine protease and are now in late phase 3 development include telaprevir and boceprevir. Results from the phase II investigations of telaprevir in naïve HCV genotype 1 patients revealed SVR rates up to 69% after a 12-week course of telaprevir in combination with peginterferon and ribavirin followed by 12 weeks of standard peginterferon plus ribavirin therapy, thus shortening the total course of therapy to 24 weeks.97,98 Telaprevir has also demonstrated efficacy in the retreatment of genotype 1 patients who previously failed combination peginterferon and ribavirin, with reported SVR rates up to 39% in prior nonresponders and 76% in prior relapsers.99 Likewise, early reports on the efficacy of boceprevir in combination with peginterferon and ribavirin from a phase II clinical trial of treatment-naïve genotype 1 patients revealed a significant improvement over the standard of care with peginterferon alfa-2b plus ribavirin (SVR = 38%), with an SVR rate up to 75% when boceprevir was added to the standard of care.100 Several nucleoside and nonnucleoside polymerase inhibitors that target the HCV RNA-dependent RNA polymerase have also been studied, including a combination of nucleoside polymerase inhibitor (R7128) and protease inhibitor (R7227/ITMN-191) without peginterferon, which demonstrated significant antiviral potency in a recent phase I clinical trial.101
Additional agents with antiviral activity against HCV currently under study include toll-like receptor agonists, cyclophilin inhibitors, ribavirin analogues, and new antiviral agents such as nitazoxanide. In vitro studies suggest nitazoxanide may modulate host antiviral responses through activation of interferon-induced mediators such as the protein kinase activated by double-stranded RNA, resulting in antiviral activity against HCV.102 Nitazoxanide has demonstrated efficacy against HCV, particularly in genotype 4 patients, in whom SVR rates as high as 80%have been reported in combination with peginterferon and ribavirin.103,104
¿ Conclusion
Although the treatment of chronic hepatitis C remains a challenge to clinicians, several recent developments in the approach to treatment have resulted in an improved ability to achieve an SVR. Despite combination therapy with peginterferon and ribavirin remains the gold standard in the treatment of chronic HCV infection, the use of optimal dosing regimens and the assessment of viro-logic responses during a course of therapy allows for individualization of therapy and further improvements in outcomes using current therapy. Several new DAA agents against HCV are currently under investigation. These newer agents used in combination with peginterferon and ribavirin will allow a shorter 24-week course of therapy in the majority of patients with genotype 1 infection, saving costs and reducing the morbidity of therapy. In addition, the use of a DDA agent in conjunction with interferon-based therapy has been shown to result in a major improvement in SVR rates in patients who have failed prior therapy. It is estimated that licensure of telaprevir and boceprevir will occur in late 2011, which in combination with routine use of IL28B testing will lead to the development of much more complex guidelines for the treatment of chronic hepatitis C. All of these advances in therapy resulting in higher SVR rates will have a major impact on the liver-related morbidity and mortality associated with chronic hepatitis C, including reduction in the rates of progression to cirrhosis and the development of HCC, with prolongation of life.
Abbreviations used:
AASLD = American Association for the Study of Liver Diseases
AGA = American Gastroenterological Association
ALT = alanine aminotransferase
Anti-HCV = antibody to hepatitis C virus
BMI = body mass index
cEVR = complete early virologic response
DAA = direct acting antiviral
ETR = end of treatment response
EVR = early virologic response
HBV = hepatitis B virus
HCV = hepatitis C virus
HCV RNA = hepatitis C virus ribonucleic acid
NIH = National Institutes of Health
PEG = polyethylene glycol
pEVR = partial early virologic response
RVR = rapid virologic response
SVR = sustained virologic response
Correspondence: Emmet B. Keeffe, MD.
750 Welch Road, Suite 210. Palo Alto, CA 94304-1509.
Phone: 650-498-5691. Fax: 650-498-5692
E mail:ekeeffe@stanford.edu