In Mexico, complications of cirrhosis are the third leading cause of death in adult males. In recent decades, the incidence of hepatocellular carcinoma has increased worldwide. The aim of this study was to determine the characteristics of patients with hepatocellular carcinoma at two Mexican tertiary care hospitals.
Material and methodsAn observational, cross-sectional, retrospective study was conducted between January 2008 and April 2014. We described the clinical features, epidemiologic characteristics, diagnosis, and treatment of patients with hepatocellular carcinoma.
ResultsOne hundred and forty-eight patients were included. There was a predominance in males and disease manifestation in the sixth decade of life. Liver disease was associated in 87% of subjects and was mainly attributed to alcohol abuse, hepatitis C infection, and nonalcoholic steatohepatitis. Sixty percent (60%) of cases were classified as Child-Pugh stage A cirrhosis, 75.5% harbored a single tumor at diagnosis, 27.7% had normal alpha-fetoprotein values, and only 39.2% of patients with known liver disease were under a surveillance program. Tumors were larger than 5cm at diagnosis in 64.3% of patients, and well-differentiated lesions were most frequently detected. Over 70% of patients were diagnosed at a non-curative stage. By the 2014 study cutoff point, 77.7% of patients had died. Treatment was determined by the means available at each center and followed the therapeutic recommendations in international guidelines in 45.3% of cases, clearly impacting survival.
ConclusionsBetter surveillance methods are required to diagnose the disease at its early stages, but treatment still requires individual adaptation to each center's available resources.
En México, las complicaciones de la cirrosis son la tercera causa de muerte en adultos de sexo masculino. En años recientes el carcinoma hepatocelular ha demostrado incremento en su incidencia global. El objetivo de este estudio fue determinar las características del carcinoma hepatocelular en dos hospitales mexicanos de tercer nivel.
Material y métodosEstudio observacional, transversal y retrospectivo, de enero de 2008 a abril de 2014; se describen las características clínicas y epidemiológicas, así como el diagnóstico y tratamiento, de 148 pacientes con carcinoma hepatocelular.
ResultadosEl carcinoma hepatocelular se presentó predominantemente en el sexo masculino y en la sexta década de la vida, asociado a enfermedad hepática en el 87% de los casos; la etiología más frecuente fue la cirrosis secundaria a alcohol, el virus de la hepatitis C y la esteatohepatitis no alcohólica. Al diagnóstico, el 60% estaban en Child-Pugh A, el 75.5% presentaron tumor único, y el 27.7% tuvieron la alfafetoproteína normal. El 39.9% de los pacientes con enfermedad hepática conocida se encontraban bajo un programa de vigilancia. El 64.3% de pacientes tuvieron tumores más grandes de 5cm al momento del diagnóstico, y las lesiones bien diferenciadas fueron las detectadas con mayor frecuencia. Más del 70% de los pacientes se diagnosticaron en una etapa no curativa. Al llegar al punto de corte en el 2014, el 77.7% de los pacientes ya habían fallecido. El tratamiento se determinó de acuerdo con la disponibilidad de cada centro y las guías internacionales se siguieron en el 45.3% de los casos, impactando en la supervivencia.
ConclusionesSe requiere mejorar el escrutinio para detectar la enfermedad en etapas tempranas; sin embargo, se debe adaptar el tratamiento dependiendo de la experiencia en cada centro.
The incidence of hepatocellular carcinoma (HCC) has progressively increased worldwide in recent decades and the main risk factor for its development is the presence of underlying cirrhosis. Hepatitis B and/or C viral infection and alcohol abuse are among the major pathologies leading to cirrhosis, and nonalcoholic steatohepatitis (NASH) is now considered an emerging and important etiology of cirrhosis and HCC.1–3 Various algorithms have been used for the surveillance and treatment of each disease, and all are fully endorsed by several international associations.4,5 In Mexico, there is currently insufficient epidemiologic data on HCC cases and their relation to the underlying liver disease, as well as a lack of information on tumor characteristics at diagnosis, on management, and on survival. The aim of this study was to characterize a cohort of 148 patients diagnosed with HCC from two tertiary care centers in Mexico.
Materials and methodsPatients diagnosed with HCC between January 2008 and April 2014 at the following two Mexican tertiary care hospital centers were included in the study: the Unidad Médica de Alta Especialidad no. 25, Instituto Mexicano del Seguro Social, Monterrey Nuevo León (UMAE 25) (Northern Mexico) and the Instituto de Seguridad Social del Estado de México y Municipios (ISSEMyM), Toluca, Estado de México (Central Mexico). The study protocol was approved by the Ethics and Research Committee of the UMAE 25 IMSS and the Ethics Committee of the ISSEMyM.
The documented epidemiologic data included: etiology of the underlying liver disease, liver disease stage at diagnosis, and the HCC stage according to the Barcelona Clinic Liver Cancer (BCLC) staging classification. Patients were followed from January 2008 to April 2014, the study cutoff point. Surveillance consisted of liver ultrasound images obtained every 6-12 months. Alpha-fetoprotein (AFP) levels were measured at diagnosis and each group's survival was recorded. The study was observational, cross-sectional, and retrospective.
The statistical analysis was carried out employing measures of central tendency through mean, median, standard deviation, and percentage.
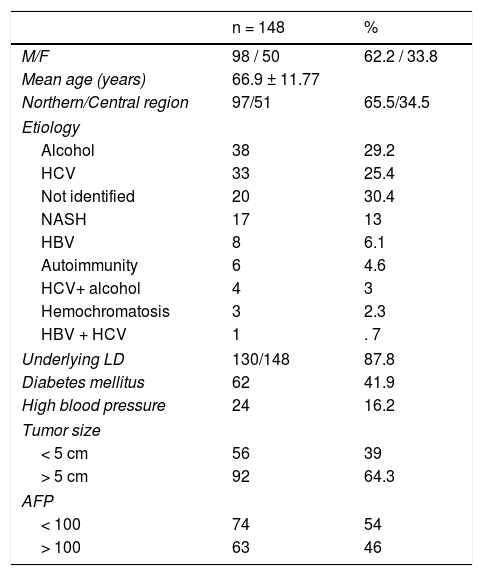
ResultsGeneral data. 148 patients were included, 98 (66.2%) were male and 50 (33.8%) were female. Their mean age was 63.9 years (SD ± 11.77, range 52-76 years). Ninety-seven patients (65.5%) were from Northern Mexico and 51 (34.5%) were from Central Mexico. Major comorbidities included type 2 diabetes mellitus in 62 (41.9%) cases and high blood pressure in 24 patients (16.2%).
Liver disease. It was possible to document underlying chronic liver disease in 130 (87.8%) patients, and the etiologies are shown in Table 1, in descending order of frequency. As expected, alcohol abuse and chronic hepatitis C virus (HCV) infection were the most frequent causes of liver disease. The “not identified” category was applied to cases with no significant history of alcohol ingestion, negative markers for viral hepatitis, and no studies confirming other possible etiologic factors. The third cause identified was NASH. Fifty-one (39.2%) of the 130 patients with underlying liver disease were followed in a surveillance program and diagnosed under that strategy. The remaining patients were diagnosed from reported symptoms and/or clinical findings associated with the tumor. Some of those patients had simultaneous diagnoses of liver disease and HCC.
Clinical and epidemiologic data in Mexican HCC patients.
| n = 148 | % | |
|---|---|---|
| M/F | 98 / 50 | 62.2 / 33.8 |
| Mean age (years) | 66.9 ± 11.77 | |
| Northern/Central region | 97/51 | 65.5/34.5 |
| Etiology | ||
| Alcohol | 38 | 29.2 |
| HCV | 33 | 25.4 |
| Not identified | 20 | 30.4 |
| NASH | 17 | 13 |
| HBV | 8 | 6.1 |
| Autoimmunity | 6 | 4.6 |
| HCV+ alcohol | 4 | 3 |
| Hemochromatosis | 3 | 2.3 |
| HBV + HCV | 1 | . 7 |
| Underlying LD | 130/148 | 87.8 |
| Diabetes mellitus | 62 | 41.9 |
| High blood pressure | 24 | 16.2 |
| Tumor size | ||
| < 5 cm | 56 | 39 |
| > 5 cm | 92 | 64.3 |
| AFP | ||
| < 100 | 74 | 54 |
| > 100 | 63 | 46 |
AFP: Alpha-fetoprotein; HBV: Hepatitis B virus; HCV: Hepatitis C virus; LD: Liver disease; NASH: Nonalcoholic steatohepatitis.
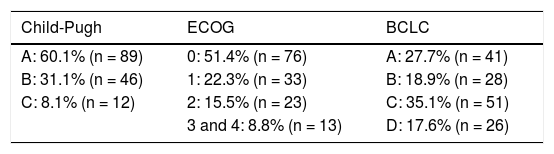
At the time of diagnosis, 89 (60.1%) were classified as Child-Pugh stage A disease, 46 (31.1%) were stage B, 12 (8.1%) were stage C, and 1 case was not classified (Table 2).
Staging at diagnosis.
| Child-Pugh | ECOG | BCLC |
|---|---|---|
| A: 60.1% (n = 89) | 0: 51.4% (n = 76) | A: 27.7% (n = 41) |
| B: 31.1% (n = 46) | 1: 22.3% (n = 33) | B: 18.9% (n = 28) |
| C: 8.1% (n = 12) | 2: 15.5% (n = 23) | C: 35.1% (n = 51) |
| 3 and 4: 8.8% (n = 13) | D: 17.6% (n = 26) |
BCLC: Barcelona Clinic Liver Cancer classification; ECOG: Eastern Cooperative Oncology Group.
Tumor characteristics. At diagnosis, 108 (75.5%) cases had a single localized tumor, whereas 39 (27.2%) patients harbored multiple or diffuse lesions. Tumors were smaller than 5cm at diagnosis in 56 (39%) patients and larger than 5cm in 92 (64.3%) cases (* n = 147, one missing value).
AFP was determined in 145 (97%) patients, with a mean level of 8,271.5 ng/ml (range: 0.83 to 431,880 ng/ml). The AFP value at diagnosis was normal in 41 (27.7%) patients and above 20 ng/ml in 70.3% (n = 104).
A liver biopsy was performed in most patients to establish a tissue bank for future research. Ninety-four patients underwent liver biopsy, and the grade of histopathologic differentiation was established in 47 cases: the tumor was well-differentiated in 25 (16.9%) cases, moderately differentiated in 18 (12.2%) and poorly differentiated in 4 (2.7%) patients. No tumor seeding was documented in the biopsies. Three patients had fibrolamellar HCC, with the following salient clinical features: a) a 46-year-old man with cirrhosis secondary to hepatitis B infection and a diffuse tumor that progressed and was not treated, due to its advanced stage; b) a 23-year-old man with no concomitant liver disease and a 15-cm tumor that was surgically resected; and c) a 23-year-old woman with no concomitant liver disease and a 6.8-cm tumor that was surgically resected.
Thirty-five patients with underlying liver disease of the 51 under surveillance (35/51) (68%) had lesions smaller than 5cm in diameter, whereas 70 of all patients not under surveillance (70/97) (72.1%) had lesions larger than 5cm. The mean diameter of the tumors diagnosed at monitoring was 4.5cm, whereas that of the others was 8.8cm. BCLC staging at diagnosis was: A: 41 (27.7%) cases, B: 28 (18.9%) cases, C: 51 (35.1%) cases, and D: 26 (17.6%) cases. Performance status was determined by the Eastern Cooperative Oncology Group (ECOG) and was distributed as follows: 0: 76 (51.4%), 1: 33 (22.3%), 2: 23 (15.5%), 3: 10 (6.8%), and 4: 3 (2%) (Table 2). According to the BCLC staging classification, a total of 48 patients under surveillance were in stages A: 20, B: 15, C: 8, and D: 5. A total of 95 patients not under surveillance were classified in stages A: 20, B: 12, C: 42; and D: 21 (BCLC/surveillance classification was not available in 5 patients).
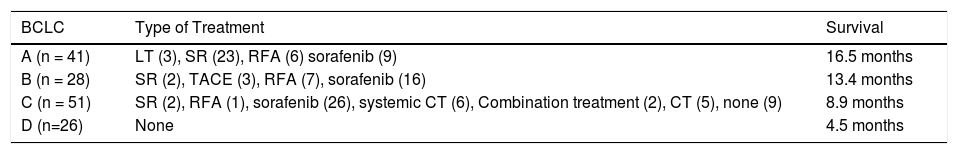
Surgical treatment was performed in 22 patients and included 19 resections and 3 liver transplants. Loco-regional treatment was performed in 30 cases, with 14 radiofrequency ablations (RFAs), 10 transarterial chemoembolizations (TACEs), combination management with RFA/TACE in 3 cases, and 3 cryoablations. Systemic therapy was delivered to 64 patients: 55 cases were treated with sorafenib and 9 patients received other systemic chemotherapies. According to the BCLC classification, 77 (45.3%) patients received the recommended treatment, whereas 54.7% received a different treatment option. Table 3 shows the treatment protocol administered in accordance with BCLC stage.
Types of treatment and survival.
| BCLC | Type of Treatment | Survival |
|---|---|---|
| A (n = 41) | LT (3), SR (23), RFA (6) sorafenib (9) | 16.5 months |
| B (n = 28) | SR (2), TACE (3), RFA (7), sorafenib (16) | 13.4 months |
| C (n = 51) | SR (2), RFA (1), sorafenib (26), systemic CT (6), Combination treatment (2), CT (5), none (9) | 8.9 months |
| D (n=26) | None | 4.5 months |
BCLC: Barcelona Clinic Liver Cancer classification; CT: Chemotherapy; LT: Liver transplant; RFA: Radiofrequency ablation; SR: Surgical resection; TACE: Transarterial chemoembolization.
Patients that progressed, regardless of their initial stage, were able to receive more than one treatment modality after restaging, if the treatments were available. At the cutoff point in April 2014, 115 (77.7%) patients had died and 33 (22.3%) were alive. The causes of death in descending order of frequency were: disease progression in 68 (45.9%) cases, sepsis in 6 (4.1%), and hemoperitoneum in 2 (1.4%) cases. Twenty-eight (18.9%) patients were lost to follow-up. Seventy percent of patients with Child-Pugh stage A disease, 84% of patients in stage B, and 100% of patients in stage C disease died.
Mortality, according to BCLC staging, was: A: 25/41 (60.9%), B: 17/28 (60.7%) C: 47/51 (92.1%), and D: 26/26 (100%). Tumor histologic differentiation analysis and the associated mortality rates were: 64% (16/25) of patients in the well-differentiated group, 94.4% (17/18) in the moderately differentiated group, and 100% (4/4) in the poorly differentiated group. At cutoff, mean survival in months according to BCLC staging and regardless of treatment received was: A: 16.5 months, B: 13.4 months, C: 8.9 months, and D: 4.5 months (Table 3).
Discussion and conclusionsIn the present series of 148 HCC cases in Mexico, male sex predominated, which coincides with the international literature, but differs from the recently published Mexican Consensus on HCC. That analysis, encompassing the years 1965 to 2013, included 372 patients and suggests a similar prevalence between the two sexes.6 The trend for HCC to appear around the sixth decade of life continues, and the main risk factor for its development is underlying liver disease, present in 87% of cases. The primary etiologic factors in our cohort were HCV and alcohol abuse, concurring with the leading causes of cirrhosis reported in Mexico.7 In light of new epidemiologic trends, non-alcoholic fatty liver disease (NAFLD) has been identified as a third cause. In fact, its prevalence is striking and it frequently coexists with diabetes mellitus and high blood pressure, at rates similar to those described in adults in the 2012 Mexican National Health Survey.8
Twenty-five percent (25%) of tumors in the present analysis were initially detected as multiple or diffuse lesions and AFP levels were normal in 27.7% of cases at diagnosis, as was also described by Arrieta et al.9 In our study, 44 patients fulfilled the Milan criteria at diagnosis, but only 3 patients had transplantation, all in Monterrey, Mexico. The remaining patients did not undergo transplantation because the procedure could not be performed as recommended by the guidelines of both centers, due to a lack of resources.
The greater mortality rate in our patients, despite the earlier BCLC staging, and compared with other case series, could be a reflection of the scant availability of treatment modalities (i.e., transplantation) at the two centers involved, as well as secondary causes, such as perforated peptic ulcer, pneumonia, sepsis, and upper gastrointestinal bleeding. In addition, our study cutoff point was in 2014, precluding a complete and adequate overview of mortality.
Tumor differentiation grade has been described as an independent prognostic factor. Tamura et al. analyzed the impact of differentiation on post-transplant patient survival.10 Our population analysis demonstrated that surveillance foments the diagnosis of smaller tumors and increases their resolution potential. However, active surveillance was carried out on only 39.2% of the patients, a problem already mentioned by several authors. Davila et al. conducted a retrospective review of 1,873 patients diagnosed with HCC in the United States and found that a formal diagnosis was made through surveillance in only 17% of the cases.11 Ideally, early detection should favor curative therapy and thus improve survival, as has previously been shown.
There are clear limitations to our study, particularly a lack of availability of recommended resources when attempting to strictly follow current international guidelines. Moreover, the use of alternative modalities available in our centers could certainly prolong the follow-up of our cohort. Several patients presented with symptoms leading to the simultaneous diagnoses of HCC and cirrhosis. This could be a failure in the system of early detection of at-risk patients and/or a lack of knowledge of the surveillance recommendations for that population. In our group, over 70% of patients were diagnosed in non-curative stages, that is, at BCLC stages B, C, and D.
Treatment was provided based on the resources available at each center. The surgical approaches of transplantation and resection were performed at the hospital center in Northern Mexico, on a limited number of patients. RFA, TACE, and the administration of sorafenib were carried out at the center in Central Mexico.
Adherence to the BCLC treatment recommendations had to be adapted in accordance with availability. This is not an uncommon reality, as documented by the BRIDGE study group.12 They included over 5,000 patients in BCLC stage C (after sorafenib approval) from 19 countries in a longitudinal study conducted on a multiregional cohort and reported that less than 1% of the population received treatment in accordance with the international guideline recommendations. As in our cohort, alternative therapies were often used. Surprisingly, the overall survival of the BRIDGE cohort was 23 months, a clear dissociation from expected survival in patients with stage C disease. Nevertheless, that strategy is not new, given that a group of experts has suggested that intermediate and advanced stages must be “subclassified” to individualize therapy and consequently improve survival.13,14 Thus, an exponential amount of information and new protocols are being published, evaluating combination therapy at those stages. They include post-resection sorafenib,15 TACE and RFA,16,17 TACE and sorafenib,18,19 and RFA plus sorafenib,20 among others.
In our group of patients with HCC, male sex was predominant and patient age was similar to that previously reported in the international literature. The frequency of underlying liver disease was high and etiologies were similar to those in previous Mexican studies on cirrhosis. Diagnosis is still made at advanced disease stages in which curative therapy is no longer an option. Our limited availability of treatment strategies, such as transplantation and resection, clearly impact survival. Therefore, the use of combination treatment strategies, according to their availability at each center, is crucial.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Financial disclosureNo financial support was received in relation to this study/article.
Conflict of interestThe authors declare that there is no conflict of interest.
Please cite this article as: Cisneros-Garza LE, González-Huezo MS, López-Cossio JA, Kuljacha-Gastelum AL. Caracterización del carcinoma hepatocelular en México. Revista de Gastroenterología de México. 2018;83:223–227.