Barrett's esophagus is a condition that predisposes to esophageal adenocarcinoma. Our aim was to establish the prevalence of Barrett's esophagus at our center, as well as determine its associated factors.
Materials and methodsWe retrospectively assessed the endoscopic reports of 500 outpatients seen at our Gastroenterology Service from November 2014 to April 2016. We determined the prevalence of Barrett's esophagus and analyzed the demographic, clinical, and endoscopic findings associated with that pathology.
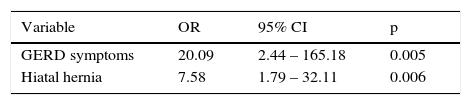
ResultsThe prevalence of Barrett's esophagus was 1.8%. The mean age of the patients with Barrett's esophagus was 58.7 years (range: 45-70) and there was a predominance of men (66%). In the subgroup of patients with symptoms of gastroesophageal reflux (n=125), Barrett's esophagus prevalence was 7.2%. In the multivariate analysis, the factors that were independently associated with Barrett's esophagus were gastroesophageal reflux (P=.005) and hiatal hernia (P=.006).
ConclusionsThe overall prevalence of Barrett's esophagus was 1.8% in our population, with a prevalence of 7.2% in patients that had symptoms of gastroesophageal reflux.
El esófago de Barrett es una condición que predispone al adenocarcinoma esofágico. Nuestro objetivo fue establecer la prevalencia de esófago de Barrett en nuestro centro, así como los factores asociados a esta condición.
Material y métodosEvaluamos retrospectivamente los reportes de 500 endoscopias superiores de pacientes ambulatorios de nuestro Servicio de Gastroenterología entre noviembre del 2014 y abril del 2016. Se determinó la prevalencia de esófago de Barrett y se analizaron los datos demográficos, clínicos y endoscópicos asociados a esta patología.
ResultadosLa prevalencia de esófago de Barrett fue del 1.8%. La edad media en los pacientes con esófago de Barrett fue de 58.7 años (rango: 45-70), con predominancia del sexo masculino (66%). En el subgrupo de pacientes con síntomas de reflujo gastroesofágico (n=125) la prevalencia de esófago de Barrett fue del 7.2%. En el análisis multivariado los factores asociados a esófago de Barrett de forma independiente fueron: síntomas de reflujo gastroesofágico (p=0.005) y hernia hiatal (p=0.006).
ConclusiónLa prevalencia global de esófago de Barrett es del 1.8% en nuestra población, con una prevalencia del 7.2% en pacientes con síntomas de reflujo gastroesofágico.
Barrett's esophagus (BE) is a consequence of chronic gastroesophageal reflux disease (GERD) that predisposes to the development of esophageal adenocarcinoma (EAC).1–4 BE is defined as the presence of columnar metaplasia of at least 1cm that replaces the normal stratified squamous epithelium of the distal esophagus.2 The increase in the incidence of EAC over the past decades,5,6 as well as its poor prognosis with a 5-year survival<15%,7 has motivated the implementation of screening programs with the aim of detecting EAC in early stages and improving survival.8,9
The prevalence of BE is variable, depending on the population and the definition used. In the general population, the prevalence of EB is 1.6%,10 whereas in the population with upper gastrointestinal symptoms, it was reported from 2.411 to 4.4%.12 In Mexico, studies conducted more than a decade ago reported a prevalence of 0.2613 to 9.2%.14 A recent study from an endoscopy referral center in Mexico City reported a BE prevalence of 0.96%.15 However, these studies have an important variation in their methodology for obtaining biopsies. Thus, it is necessary to know the epidemiology of this entity based on current guideline definitions and recommendations,2 in order to establish a screening and surveillance plan in our population.
In this descriptive and observational study, our primary aim was to determine the prevalence of BE in an outpatient population seen at our Gastroenterology Clinic, and identify the risk factors associated with this entity.
Materials and methodsWe conducted a retrospective and descriptive study, reviewing the medical records of patients seen at the outpatient clinic of the Gastroenterology Service of the Hospital Universitario “Dr. José E. González” that underwent upper gastrointestinal endoscopy between November 2014 and April 2016. Information regarding patient age and sex, endoscopy indication, and endoscopic study diagnoses was collected. All the patients underwent high resolution endoscopy with the FUJI-EG-590ZW endoscope (Tokyo, Japan).
Cases of suspected BE were identified based on the presence of a salmon-colored mucosa of more than 1cm proximal to the gastroesophageal junction seen during the endoscopic study. The gastroesophageal junction was defined as the anatomic region where the distal extension of the esophagus comes in contact with the proximal gastric fold.2 The definitive diagnosis of BE was based on the presence of endoscopic findings compatible with columnar epithelium in the distal esophagus and confirmed by the presence of specialized intestinal metaplasia on biopsies. Finally, BE was characterized as short-segment (< 3cm) or long-segment (≥ 3cm) BE.16
We obtained biopsies in all suspicious cases of BE, according to the Seattle protocol.17 We also took biopsies from the areas suspicious for dysplasia, following current guidelines.2 We confirmed the definitive diagnosis of BE based on the histopathologic reports. The same gastrointestinal pathologist analyzed the histopathologic samples. In cases of BE with dysplasia of any grade, we consulted a second pathologist for the definitive diagnosis.
In addition, we determined the presence of esophagitis, which was graded using the Los Angeles classification.18 The presence of a hiatal hernia was established endoscopically by observing the discordance between diaphragmatic impingement and the esophagogastric junction.19 The presence of gastroesophageal reflux symptoms was defined as heartburn and regurgitation within the last 3 months.
Statistical analysisStatistical analysis was performed with the SPSS Statistics Version 20.0 (IBM Corp, Armonk, N.Y., USA) program. We analyzed the patient baseline characteristics through descriptive statistics (absolute values, percentages, means and standard deviation). We determined the distribution of the variables with the Kolmogorov–Smirnov test. In the comparative analyses, we used the χ2 test for the categorical variables and Student's t test for the continuous variables. A multivariate analysis was performed to determine the variables independently associated with the risk of BE. We determined the odds ratio and 95% confidence interval of the variables of interest. A p value<0.05 was considered statistically significant.
ResultsWe analyzed a total of 500 patients, of which 312 were women (62.4%) and 188 were men (37.6%), with a mean age of 54±16.5 years. The main indications for upper gastrointestinal endoscopy were dyspepsia in 131 patients (26.2%), gastroesophageal reflux symptoms in 125 (25%), unexplained anemia in 62 (12.4%), dysphagia in 52 (10.4%), and weight loss in 45 (9%).
The main endoscopic diagnoses were: gastritis in 296 (59.2%), hiatal hernia in 83 (16.6%), duodenitis in 40 (8%), esophagitis in 38 (7.6%) (Grade A: 23, Grade B: 8, Grade C: 2, Grade D: 5), gastric ulcer in 17 (3.4%), esophageal varices in 11 (2.2%), and duodenal ulcer in 9 (1.8%). Two patients had an esophageal tumor with a histopathologic diagnosis of EAC and poorly differentiated carcinoma.
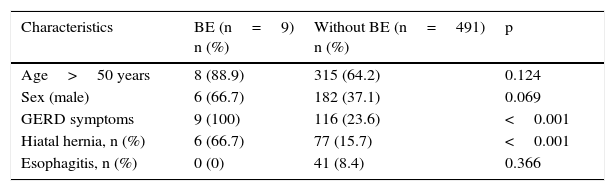
Based on the endoscopic findings, 15 patients (3%) were classified as probable BE. Of those 15 patients, 9 were histologically confirmed as BE (specialized intestinal metaplasia with the presence of goblet cells), resulting in an overall prevalence of 1.8%. Of the 9 cases of BE, 6 were classified as long-segment and 3 as short-segment. Only one case out of the 9 patients with BE presented high-grade dysplasia (11.1%).
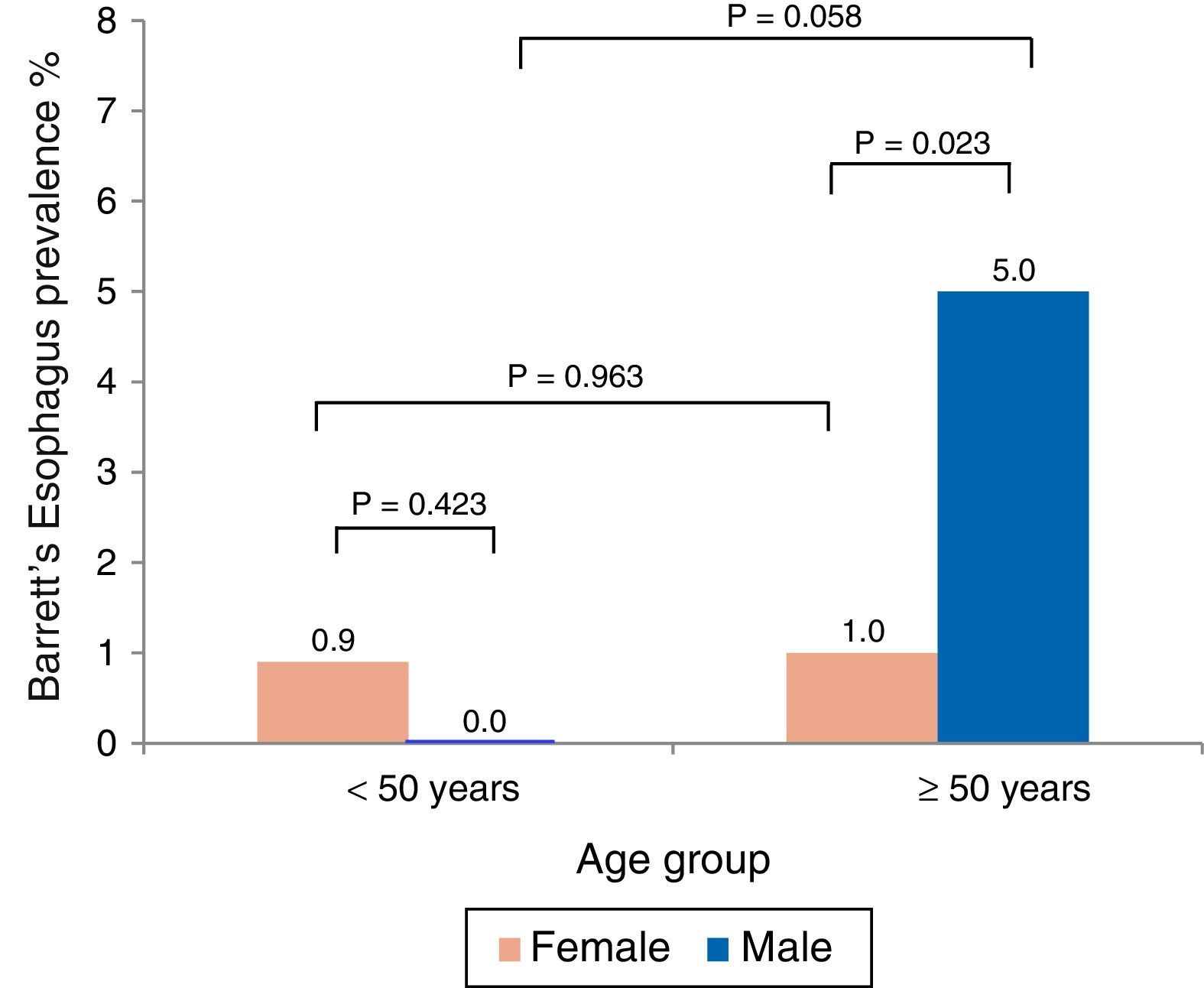
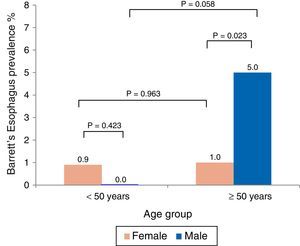
The mean age of the BE patients was 58.7±7.5 years and there was a predominance of men (66%). Analysis by age and sex showed that BE was more prevalent in men over 50 years of age (fig. 1). Likewise, BE prevalence in the subgroup of patients with symptoms of gastroesophageal reflux (n=125) was 7.2%.
In the univariate analysis, the presence of symptoms of gastroesophageal reflux (p<0.001) and hiatal hernia (p<0.001) was associated with BE (table 1). In the multivariate analysis, symptoms of gastroesophageal reflux and hiatal hernia remained independently associated with the presence of BE (table 2).
Clinical characteristics of patients with Barrett's esophagus.
| Characteristics | BE (n=9) n (%) | Without BE (n=491) n (%) | p |
|---|---|---|---|
| Age>50 years | 8 (88.9) | 315 (64.2) | 0.124 |
| Sex (male) | 6 (66.7) | 182 (37.1) | 0.069 |
| GERD symptoms | 9 (100) | 116 (23.6) | <0.001 |
| Hiatal hernia, n (%) | 6 (66.7) | 77 (15.7) | <0.001 |
| Esophagitis, n (%) | 0 (0) | 41 (8.4) | 0.366 |
BE: Barrett's esophagus; GERD: gastroesophageal reflux disease.
The current reported prevalence of BE is variable, depending on the population and the definition used.2,20 The prevalence in the population seen at our Gastroenterology Clinic was 1.8%. Ours is the only study reporting on the prevalence of BE in the Mexican Northeastern population in the last decade and we found it to be in line with the prevalence reported worldwide.10
In 2008, Fan and Snyder conducted a retrospective study in the United States evaluating the medical records and endoscopic reports of 4,500 patients. They reported a prevalence of BE of 4.4% and 1.5%, in those with and without gastroesophageal reflux symptoms, respectively12. In Mexico, the prevalence of BE is not clear. The data reported more than a decade ago vary widely, with a prevalence of 0.2613 to 9.2%.14 In the study carried out by Pena Alfaro et al.,13 which considered BE as the presence of intestinal metaplasia with goblet cells, they did not specify what type of biopsy protocol was used. Trujillo-Benavides et al.14 based their results on a small population (n=109) and the methodology for obtaining biopsies was also variable. Bernal-Mendez et al.15 presented an abstract in the Digestive Disease Week in 2015. They reviewed 43,639 endoscopies over a 10-year period from a referral center in Mexico City, identifying 420 patients with a histologic diagnosis of BE (9.6 cases/1,000 endoscopies), but they did not specify the biopsy protocol used either.
There is controversy in regard to BE diagnosis, given that the definition used in Great Britain20 establishes only the presence of metaplasia as a condition for the diagnosis of BE, contrary to the American guidelines, in which the presence of intestinal metaplasia with goblet cells is required to make the diagnosis.2
Symptoms of gastroesophageal reflux have been identified as the main risk factor associated with BE (OR: 12, 95% CI: 7.64-18.7),21 with a high prevalence in the Mexican population (19.6-40%), compared with reports in the United States (18.1-27.8%) and Europe (8.8-25.9%).22 In our study, 25% of the patients presented with gastroesophageal reflux symptoms, which was also the main risk factor associated with BE (OR: 20.09, 95% CI: 2.44-165.18). The prevalence of BE in patients with gastroesophageal reflux symptoms in our study was 7.2%.
In connection with GERD, it has been observed that patients with short-segment BE do not present with gastroesophageal reflux symptoms. Likewise, up to 40% of patients with EAC do not report a previous history of GERD.23 A study using esophageal impedance demonstrated an increase in acid and non-acid reflux episodes in patients with short-segment and long-segment BE, compared with healthy individuals.24 In our study, 100% of the patients with short and long-segment BE had reflux symptoms. However, a meta-analysis assessing 26 studies showed a significant symptomatic association with BE in those patients with long-segment BE (OR: 4.92, 95% CI: 2.01-12.0, p=0.30), but not in those with short-segment BE (OR: 1.15, 95% CI: 0.763-1.73, p=0.84).25 In this regard, acid exposure in the most distal part of the esophagus is a theory proposed to explain the development of short-segment BE, a phenomenon demonstrated in healthy individuals with no endoscopic evidence of esophagitis or hiatal hernia.26
Current clinical guidelines recommend screening patients with chronic GERD and additional risk factors, such as obesity, smoking, age>50 years, male sex, etc.2 Over the past decades, a screening and surveillance plan has been implemented in patients with GERD in search of BE, with the main purpose of preventing death by EAC, through early-stage detection of neoplasia.27 However, the quality of available evidence on the effectiveness of this strategy is not conclusive.8,9 On the other hand, the increase in the incidence of EAC in the last decades,28 the low incidence of EAC in patients with BE without dysplasia reported in recent studies,29 as well as the low frequency of BE (<10%) in patients with EAC,30,31 raise doubts as to the cost-effectiveness of current screening and surveillance strategies.
In our study, we found only one patient with dysplasia (high grade) originating in BE (11.1%), which is lower than the previously reported prevalence of dysplasia/cancer in BE in Mexico (15.1-19.3%).13,15 Bernal-Mendez et al.15 reported that 81 out of 420 patients with BE presented with some degree of dysplasia or cancer (11.4% with low-grade dysplasia, 4.8% with high-grade dysplasia, and 3.1% with esophageal cancer). This information plus the knowledge of BE epidemiology at our center could help to develop cost-effective strategies for the identification and surveillance of patients with BE.
In conclusion, the prevalence of BE at our Gastroenterology Clinic is 1.8%, with a prevalence of 7.2% in patients with symptoms of gastroesophageal reflux. Multicenter studies are required to more clearly determine the epidemiology of BE in Mexico, after which a cost-effective strategy for BE screening and surveillance can be developed.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Financial disclosureNo financial support was received in relation to this article.
Conflict of interestThe authors declare that there is no conflict of interest.
The authors wish to thank Sergio Lozano-Rodriguez, M.D. for his help in translating and editing the manuscript.
Please cite this article as: Herrera Elizondo JL, Monreal Robles R, García Compean D, González Moreno EI, Borjas Almaguer OD, Maldonado Garza HJ, et al. Prevalencia de esófago de Barrett: estudio observacional en una clínica de gastroenterología. Revista de Gastroenterología de México. 2017;82:296–300.