Graft-versus-host disease (GVHD) is a common multisystemic complication of allogeneic hematopoietic cell transplantation. The most frequent presentations of graft-versus-host disease involve the skin, the gastrointestinal tract, and the liver. The aim of the present study was to know the frequency of gastrointestinal tract and liver GVHD and the characteristics of disease presentation in pediatric patients that underwent hematopoietic stem cell transplantation (HSCT) at a tertiary care hospital center in Mexico City.
Material and methodsA retrospective study was carried out, utilizing the case records of patients that underwent HSCT in 2015, to determine the frequency of GVHD in pediatric patients at a Mexican tertiary care hospital center.
ResultsIn 2015, 16 HSCT were performed, 11 of which were carried out in males (68%). Only 3 patients developed graft-versus-host disease (18.7%). One patient presented with skin and liver GVHD and 2 patients presented with gastrointestinal tract and liver GVHD, which was the most frequent type.
ConclusionsHSCT is still an uncommon procedure in Mexico and there is a lower frequency of gastrointestinal tract and liver GVHD than that reported in other studies. Most certainly, there will be an increase in this type of patient and risk factors in the Mexican population must still be determined to help predict the onset of GVHD.
La enfermedad de injerto contra huésped (EICH) es una complicación común y multisistémica del trasplante alogénico de células hematopoyéticas. La presentación clínica más frecuente de la EICH involucra la piel, el tracto gastrointestinal y el hígado. El objetivo de este estudio fue conocer la frecuencia y características de presentación de EICH gastrointestinal y hepático en pacientes pediátricos receptores de trasplante de células progenitoras hematopoyéticas (TCPH), que son atendidos en una institución pediátrica de tercer nivel de atención en México.
Material y métodosSe realizó un estudio retrospectivo de los expedientes de los pacientes receptores de TCPH durante el año 2015, para conocer la frecuencia de EICH en pacientes pediátricos de una institución de tercer nivel de atención en México.
ResultadosDurante el transcurso del año 2015 se realizaron 16 TCPH, 11 de los cuales correspondieron al sexo masculino (68%). Solo 3 pacientes desarrollaron EICH (18.7%). De estos, uno cursó con EICH hepático aislado, y 2 presentaron EICH tanto gastrointestinal como hepático, siendo esta la presentación más frecuente; mientras que solamente un paciente presentó EICH de tipo cutáneo.
ConclusionesEl TCPH es aún un procedimiento poco frecuente en nuestro medio y la frecuencia de EICH hepático y gastrointestinal es menor que la reportada en otros estudios, sin embargo, cada vez con mayor frecuencia tendremos que enfrentarnos a este tipo de pacientes. Existen factores de riesgo aún por determinar en nuestra población, factores que ayudarían a predecir la aparición de este padecimiento.
Graft-versus-host disease (GVHD) is a common, multisystemic complication of allogeneic hematopoietic cell transplantation. It occurs when the transplanted immune system cells (graft) come from an unidentified donor, recognize the transplantation recipient (host) as a stranger, and begin an immunologic reaction that causes the disease in question in the transplant recipient.1,2 The most frequent clinical presentations of GVHD involve the skin, the gastrointestinal tract, and the liver.2 Acute gastrointestinal GVHD frequently involves both the upper and lower tracts. Enteropathy usually presents with abdominal pain or diarrhea, but in some cases, can manifest as nausea, vomiting, and anorexia. Gastrointestinal GVHD diagnosis requires clinical suspicion with confirmatory histopathologic findings in endoscopic biopsy.3–4 Once the diagnosis is made, the grade of intestinal damage is evaluated based on the clinical data of the patient.5 Liver damage usually accompanies skin or gastrointestinal GVHD, and even though it can be suspected due to elevated aminotransferase levels, liver biopsy is required to document the presence of liver GVHD. It is characterized by extensive bile duct damage with atypia and degeneration, as well as lymphocytic infiltration of the ductal epithelium.6,7
The aim of the present study was to know the frequency and presentation characteristics of liver and intestinal GVHD in pediatric patients that underwent hematopoietic progenitor cell transplantation (HPCT) seen at a Mexican tertiary care hospital.
Materials and methodsA retrospective study of the case records of patients that underwent HPCT in 2015 was conducted to know the frequency of GVHD in pediatric patients seen at the tertiary care pediatric hospital, Hospital Infantil de México Federico Gómez, in Mexico City. All the patients that underwent HPCT, regardless of the underlying disease, were included in the study. There were no exclusion criteria.
The case records were retrieved from the hospital clinical archive and the data registry of the Department of Gastroenterology and Nutrition. The following data were registered on a data collection sheet: sex, age, family history, clinical variables, clinical manifestations, nutritional status, laboratory tests, imaging studies, histopathologic report, complications from GVHD, and GVHD type. The descriptive statistics used frequencies and proportions for the qualitative variables and median and interquartile range for the quantitative variables. Given the nature of the study, there were no ethical implications. No personal patient data appear in the study.
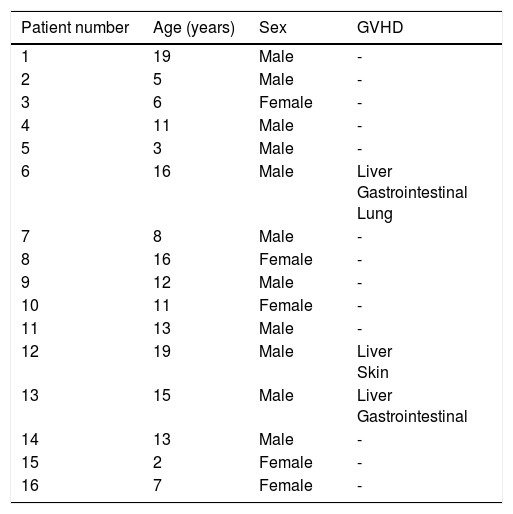
ResultsSixteen HPCTs were performed during 2015. Sixty-eight percent of them were performed in males, median patient age was 11.5 year, with an interval of 2-19 years, and an interquartile range of 9 (Table 1). Three patients developed GVHD (18.7%), of whom one presented with skin and liver graft-versus-host disease and two presented with gastrointestinal tract and liver graft-versus-host disease. Two of the patients had a history of viral infection: one with a history of hepatitis B virus infection and the other of cytomegalovirus (CMV). Two patients developed severe hematologic and infectious complications, leading to their deaths. The median length of time between HPCT and the onset of GVHD was 54 days, with a range of 113 days.
Characteristics of the patients that underwent transplantation.
| Patient number | Age (years) | Sex | GVHD |
|---|---|---|---|
| 1 | 19 | Male | - |
| 2 | 5 | Male | - |
| 3 | 6 | Female | - |
| 4 | 11 | Male | - |
| 5 | 3 | Male | - |
| 6 | 16 | Male | Liver Gastrointestinal Lung |
| 7 | 8 | Male | - |
| 8 | 16 | Female | - |
| 9 | 12 | Male | - |
| 10 | 11 | Female | - |
| 11 | 13 | Male | - |
| 12 | 19 | Male | Liver Skin |
| 13 | 15 | Male | Liver Gastrointestinal |
| 14 | 13 | Male | - |
| 15 | 2 | Female | - |
| 16 | 7 | Female | - |
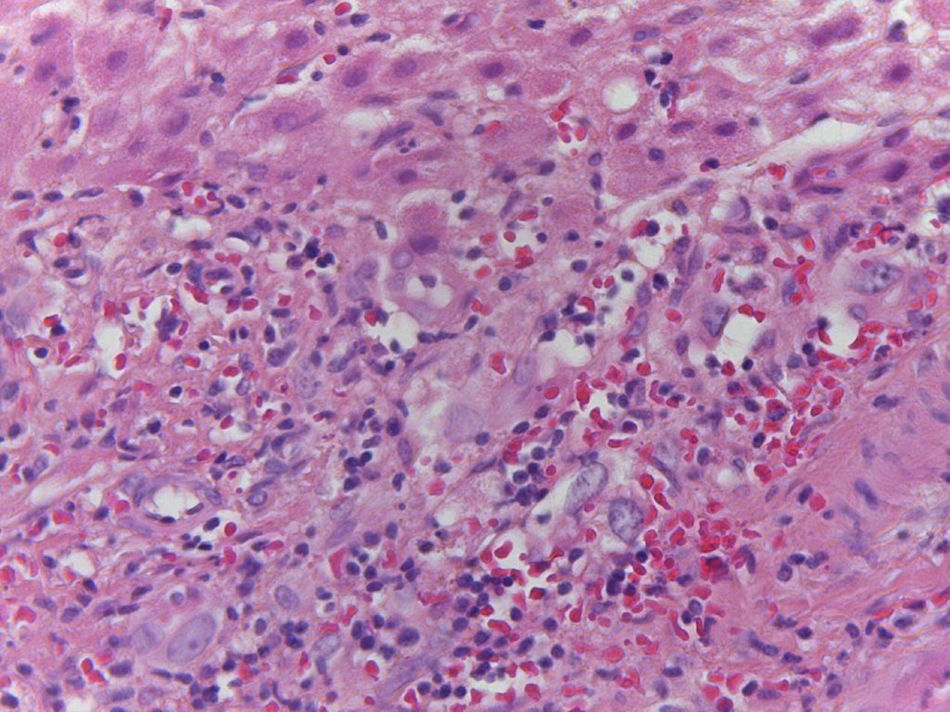
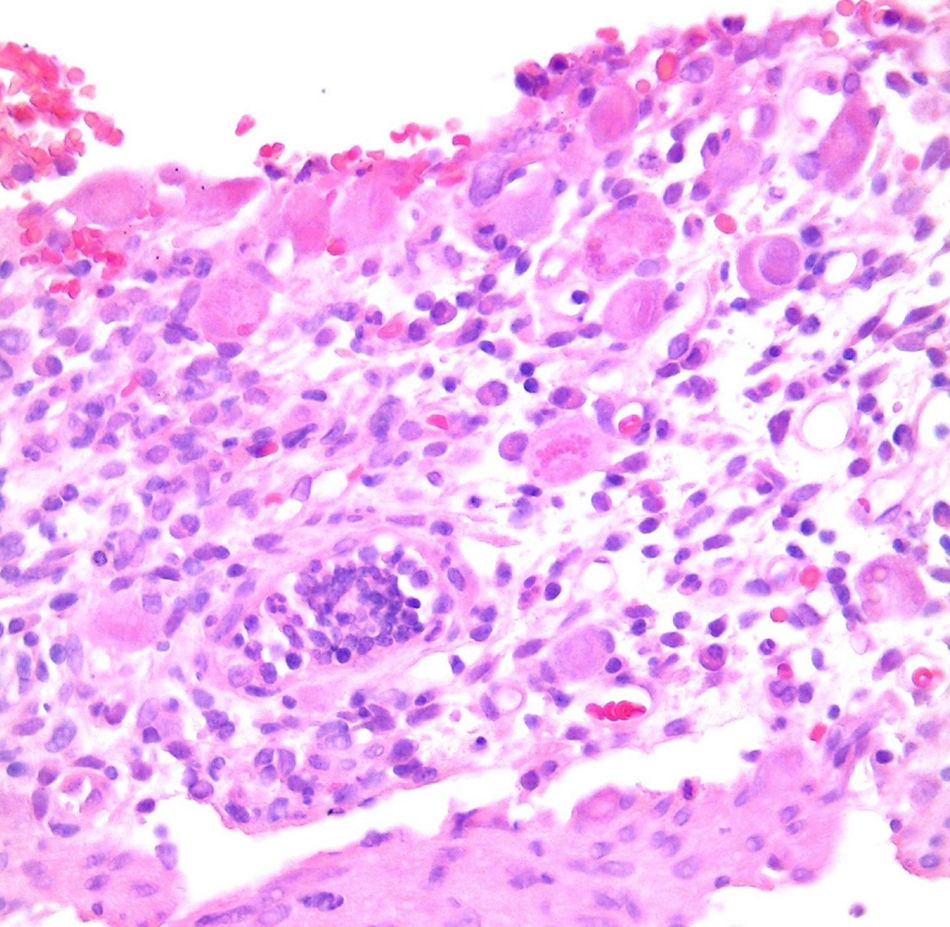
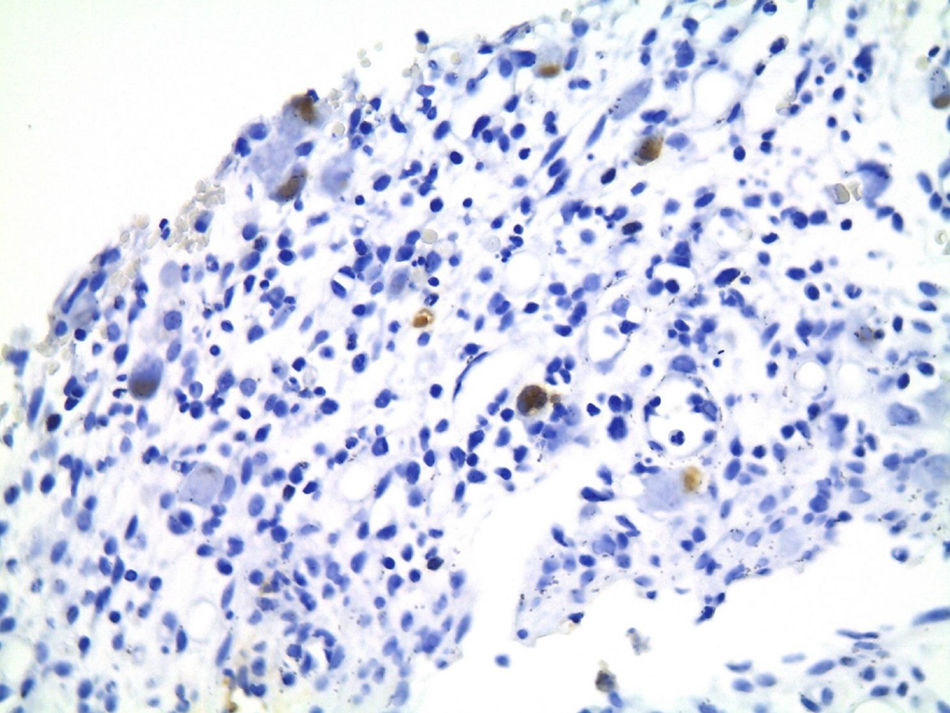
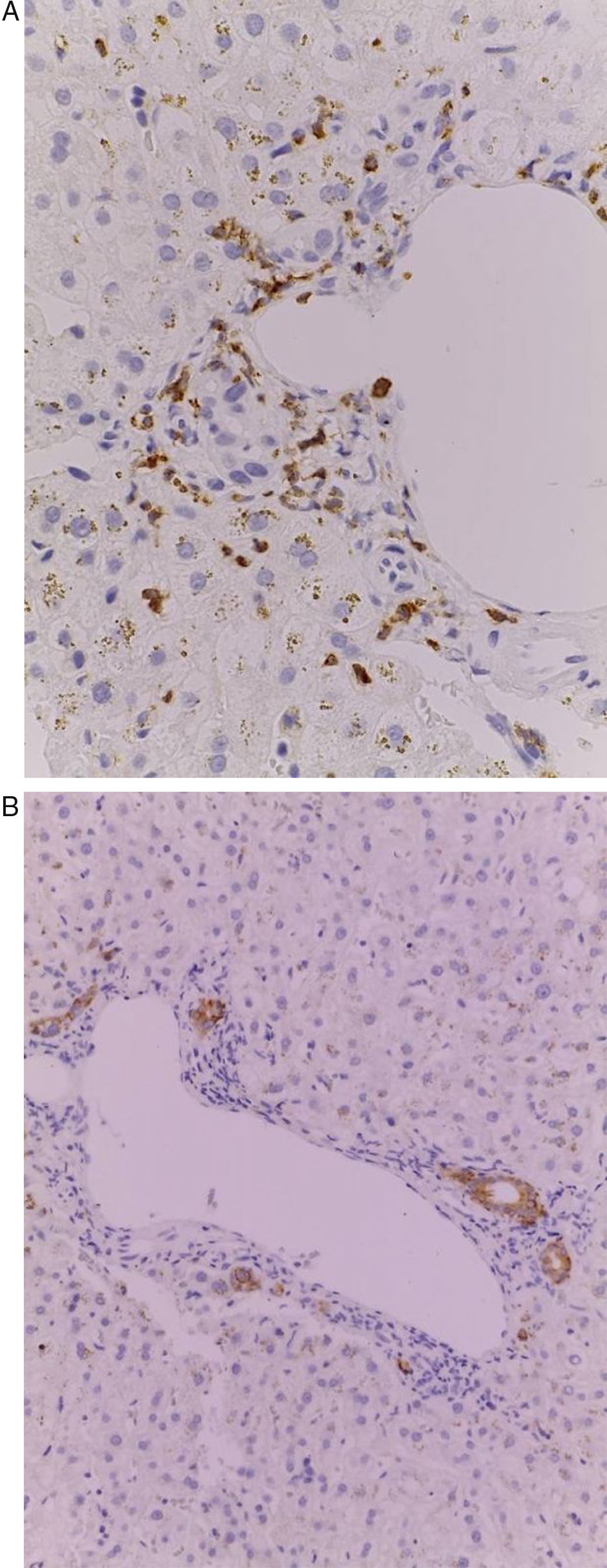
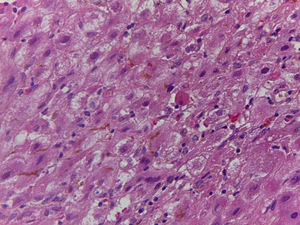
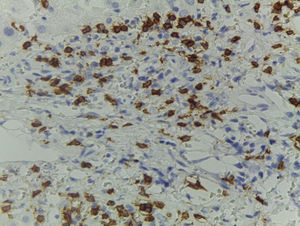
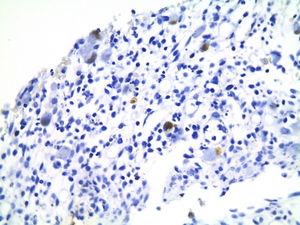
Of the three patients that developed GVHD, patient #12 (as numbered in Table 1) was a 19-year-old boy with a history of Langerhans cell histiocytosis, who later developed myelodysplastic syndrome. Successful maternal-donor HPCT was performed. Symptoms consistent with stage IV liver GVHD began on post-transplantation day 120 and included generalized jaundice, pruritus, and biochemical changes with aminotransferase levels 3-fold higher than the upper limit of normal (ULN) and increased total bilirubin of 15mg/dl, direct bilirubin of 10mg/dl, and indirect bilirubin of 5mg/dl. The patient also presented with skin GVHD. During his hospitalization, he had multiple infectious complications, nosocomial pneumonia, and septic shock that progressed to amine-refractory septic shock. The patient died due to massive pulmonary bleeding (figs. 1-3).
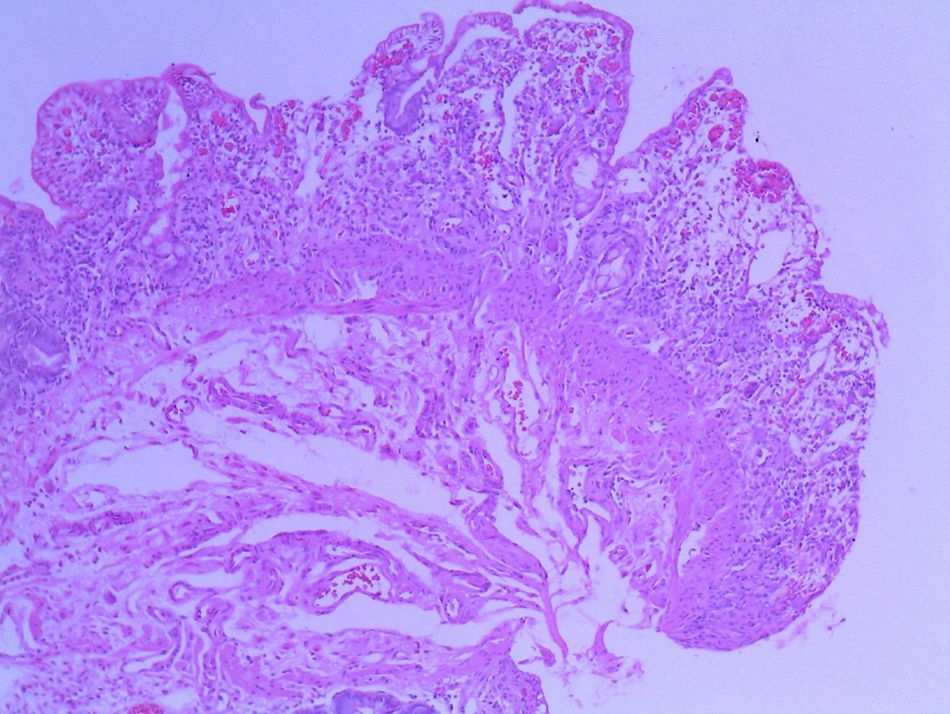
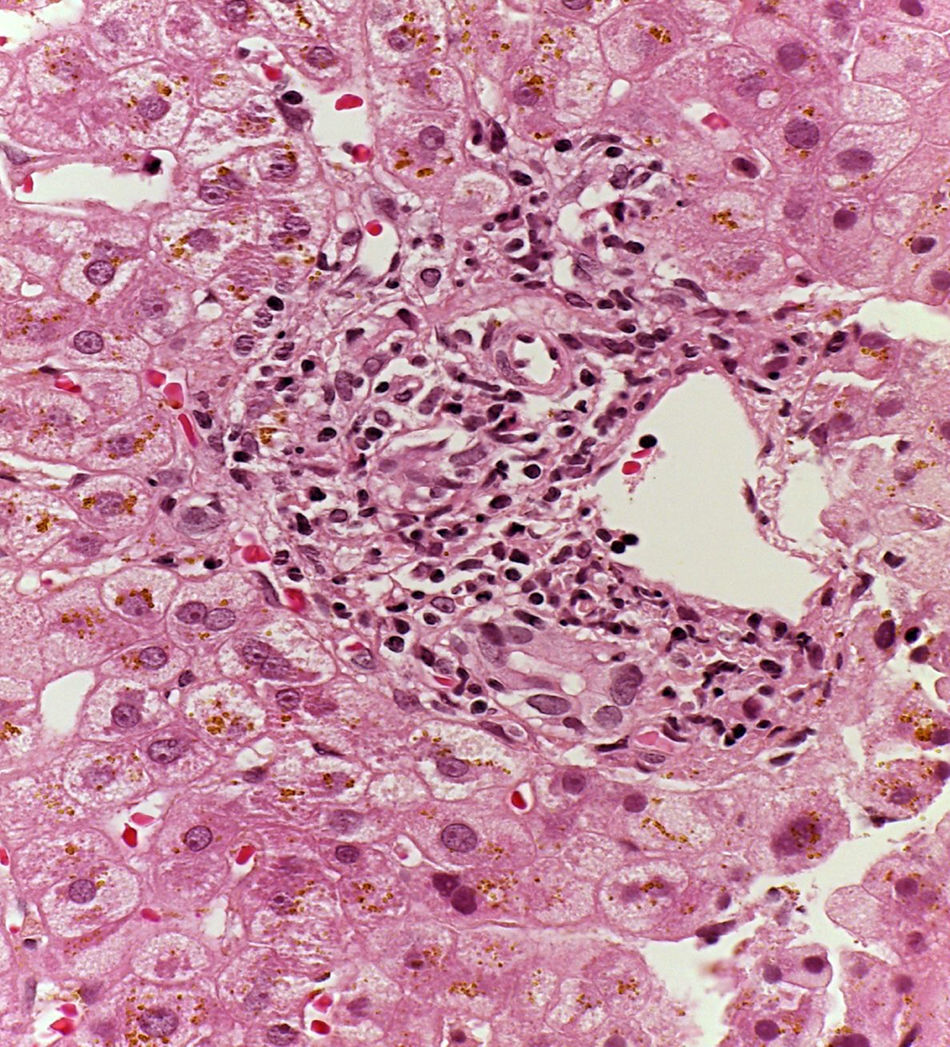
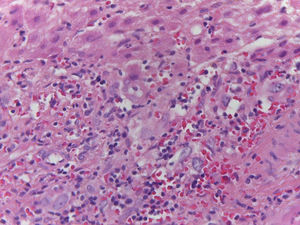
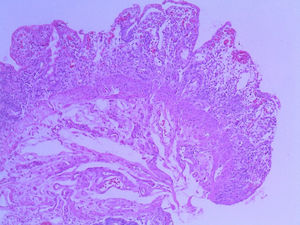
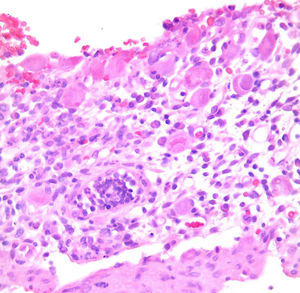
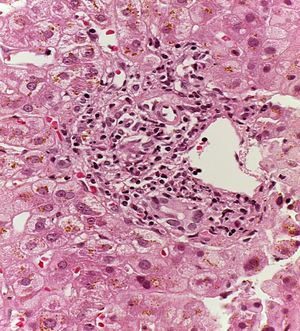
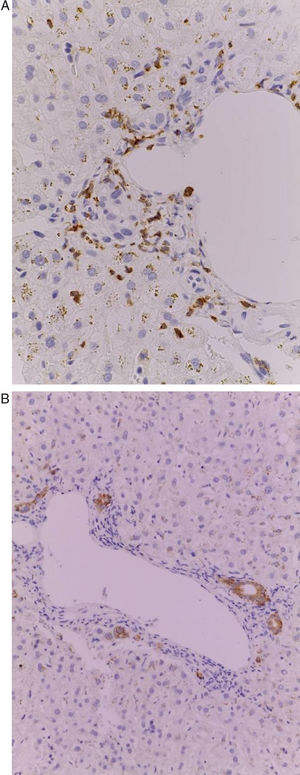
Patient #13 was a 15-year-old male with aplastic anemia. HPCT was performed and his brother was the donor. The first transplantation, with 70% chimerism, failed and a second transplantation was carried out. Grade 1 gastrointestinal GVHD presented on day 7 after the second transplant. On day 19, colonoscopy and upper GI endoscopy were performed, and the histopathologic findings were ulcerated acute colitis from CMV (figs. 4-6). He presented with multiple infectious complications that resulted in his death.
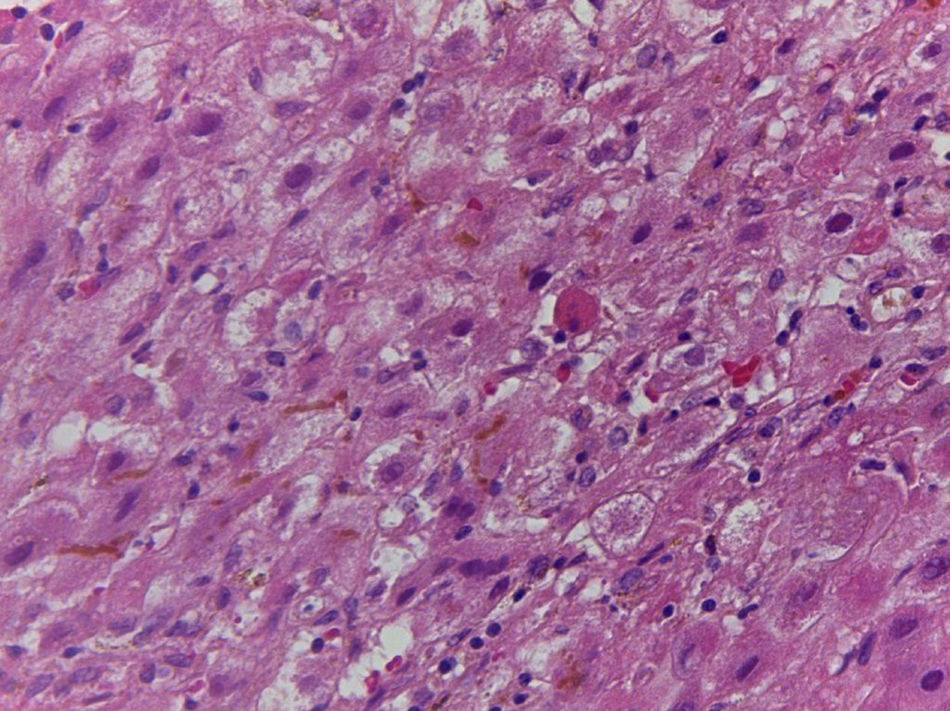
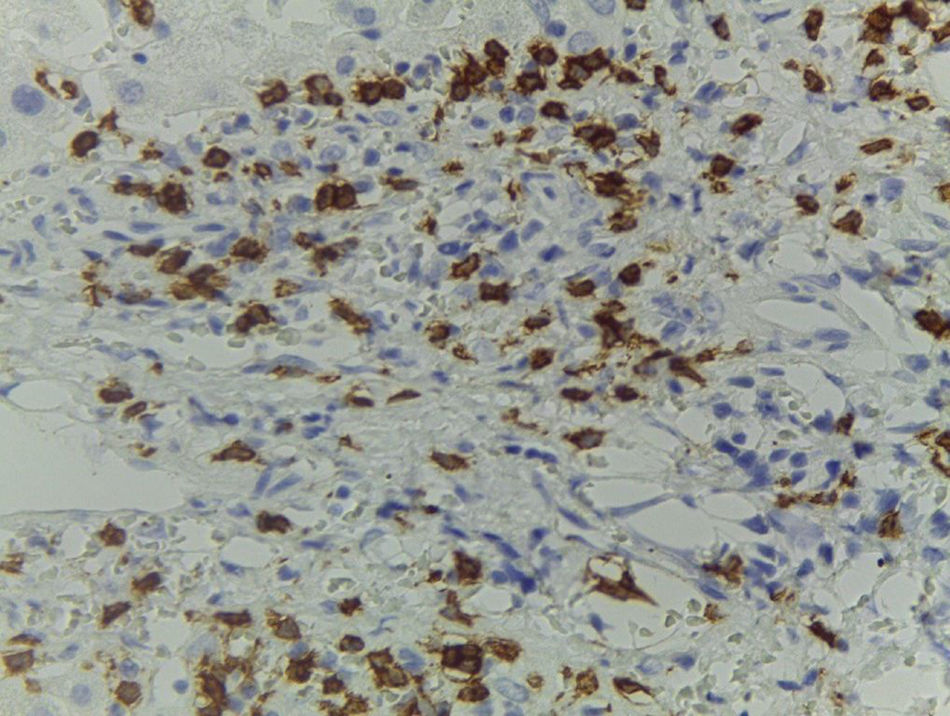
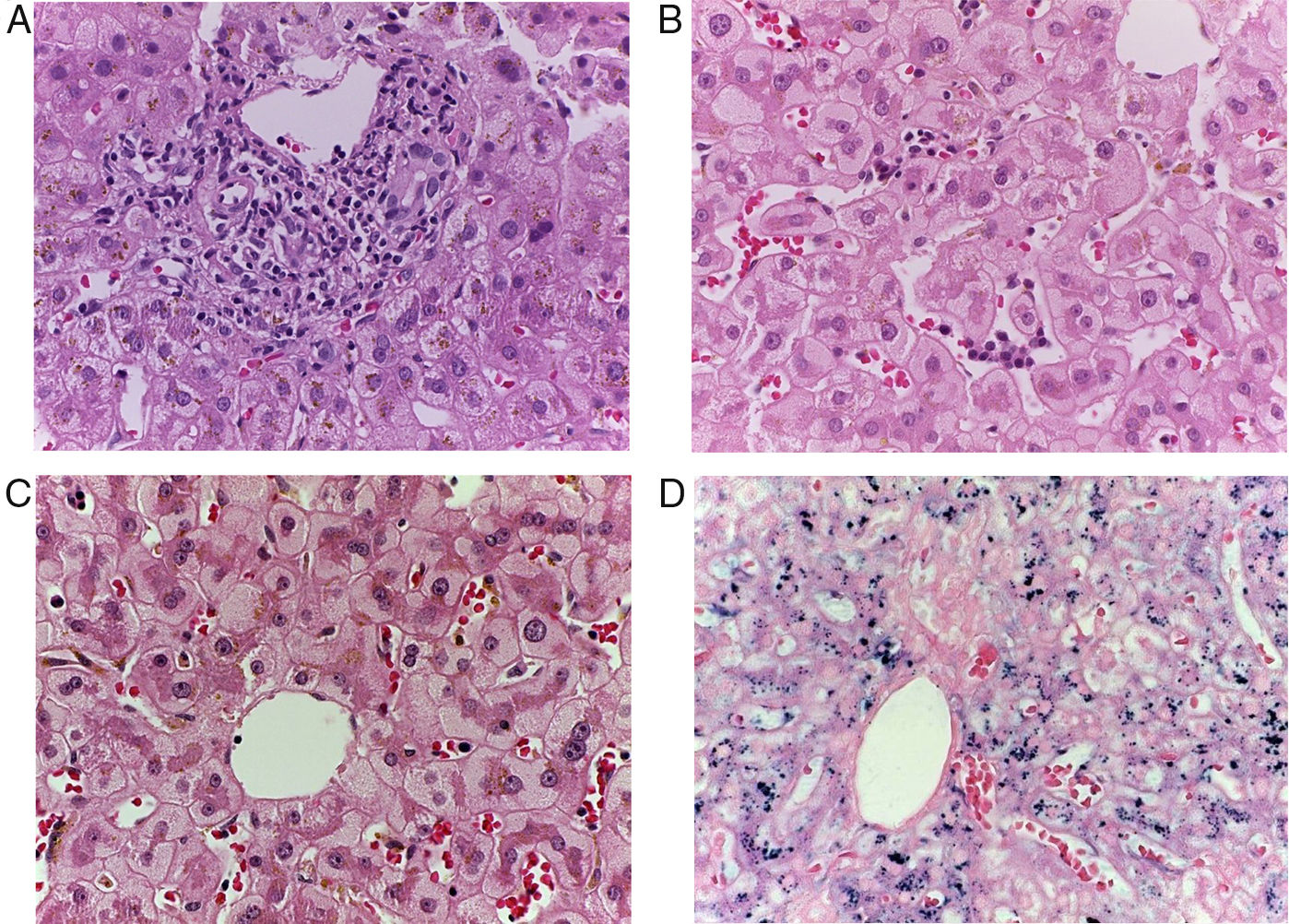
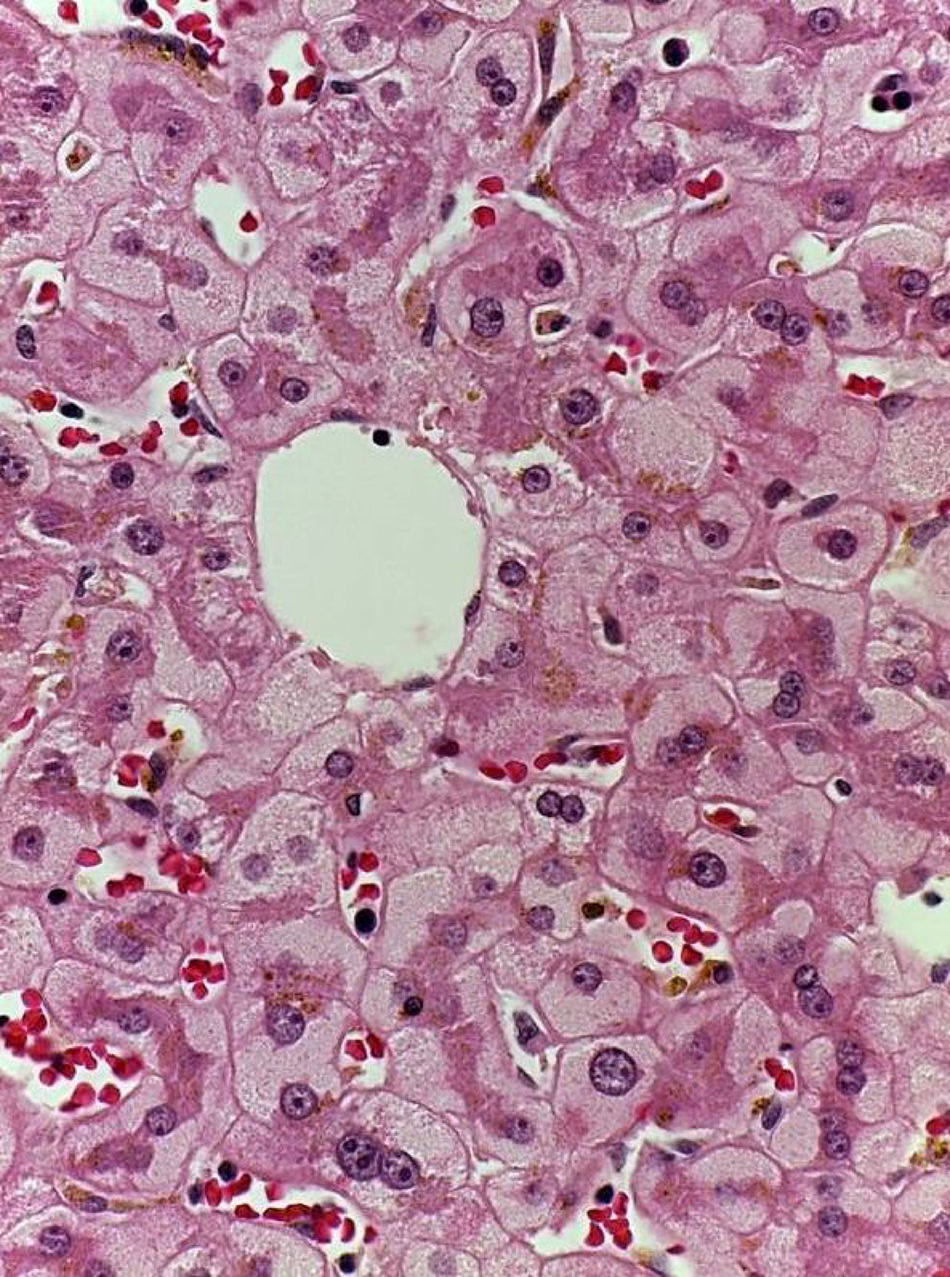
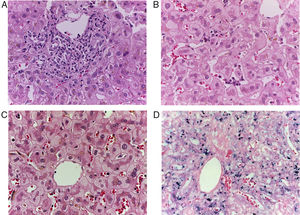
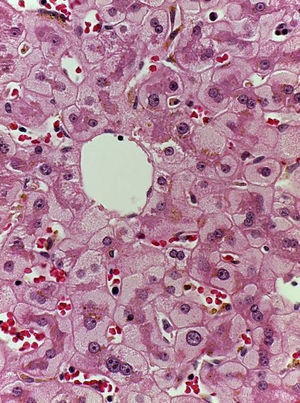
Patient #6 was a 16-year-old male with a history of M4 myeloid leukemia with 3 relapses and failed medical treatment. He received allogeneic HPCT from an unrelated donor. He presented with acute liver GVHD on post-transplantation day 37. Liver biopsy revealed WHO grade 4 hemosiderosis and changes consistent with viral infection versus reaction to medication (figs. 7-10). The patient presented with severe sepsis on day 176 but had favorable progression. He was released to his home on day 201 and continues to be under surveillance.
A) Portal lymphocytic infiltrate and bile duct damage (H&E x400). B) Focal lymphocytic infiltrate in the lobule (H&E x400). C) Perivenular hepatocytes with clear, granular cytoplasm and iron deposits (H&E x400). D) Positive Perls stain in hepatocyte cytoplasm and Kupffer cells (x400).
GVHD is an example of the main function of the immune system: to distinguish between “self” and “non-self”.8 GVHD is a complex, multifaceted process, and even though many of its mechanisms have been explained in animal models, several are still not understood. Three factors are needed for GVHD to develop: the graft must contain immunologically competent cells; the host must have antigens that do not exist in the graft, and thus seem foreign to the graft, and the host cells stimulate the graft cells through those antigens; the host must not be able to produce a reaction against the graft that lasts long enough for the graft to attack the host. GVHD is mediated by T cells. In HPCT, the primary antigenic target of tissual T cells are the major histocompatibility complexes (MHCs) of the host. However, even patients with matched MHC graft present an antigen response to minor histocompatibility complexes, which appears to the basis of GVHD development in MHC-matched patients.2,9,10
Acute GVHD is a common complication of allogeneic hematopoietic cell transplantation that classically presents in the early post-transplantation period, before 100 days. However, that definition has been modified, and the clinical presentation of classic acute GVHD symptoms is now more important than the time of presentation onset.11 Only one of our patients presented with GVHD after post-transplantation day 100.
HPCT is an advanced-specialty procedure that is increasingly being performed in the Mexican medical setting, with an improving survival rate, resulting in the augmented prevalence and incidence of GVHD, a disease with many opportunities to be analyzed. For example, in a prospective study, 27 patients with suspicion of acute gastrointestinal GVHD were endoscopically evaluated, with biopsy of the upper and lower tracts. Acute GVHD was confirmed in 18 patients (67%), whereas upper and lower diffuse intestinal damage was found in 15 patients.3 The frequency of GVHD in our study was only 18.7%, but given that HPCT is not often performed at our hospital, we must wait for a larger number of patients to establish its true incidence.
Clinically significant GVHD occurs in 20-60% of the patients that undergo HPCT, and without adequate prophylaxis with immunosuppressants before transplantation, the percentage can reach 80-100%. Survival is significantly modified in patients that present with moderate GVHD (grade II) or severe GVHD (grade III-IV). That was seen in 2 of our patients. Despite their receiving the recommended immunosuppressive prophylaxis, it was not aggressive enough to prevent GVHD.12,13 GVHD is a frequent disease in the context of HPCT. The low presentation rate in our study is most likely the reflection of the presently small number of patients at our hospital. If our sample size were larger, incidence would probably coincide with that reported in other international case series.
GVHD can affect any organ, but the skin, gastrointestinal tissue, and liver are the most prevalent sites in the majority of case series, including ours. In a study on 329 patients that were recipients of allogeneic hematopoietic progenitor cell transplantation, 110 developed grade II to IV acute GVHD, 70% of whom presented with skin involvement, 74% with gastrointestinal involvement, and 44% with liver involvement.2
Human leukocyte antigen (HLA) disparity, donor sex (female donor in a male recipient), inadequate or nonexistent prophylaxis, the conditioning regimen, and graft source are well-established risk factors for GVHD development.14
Clinical suspicion is required to accurately diagnose gastrointestinal GVHD, as well as histopathologic evaluation. Rectal biopsy, in which crypt cell necrosis with degenerative material accumulation is observed in the specimen, is the most frequently used.3,4 Crypt cell necrosis with degenerative material deposit is also observed in the histologic study of gastrointestinal GVHD and completely denuded areas of the tract with total epithelial loss can be found in severe GVHD.15 Once the diagnosis is made, the grade of intestinal damage is evaluated, based on the clinical data of the patient. One of the most important parameters is the volume of diarrheic stools per day, which is classified in 4 stages: stage 1: <10ml/kg/day, stage 2: 10-20ml/kg/day, stage 3> 30ml/kg/day, and stage 4: hematochezia, melena, mild or severe abdominal pain.5 The stools are secretory and characteristically continue during the day or night, despite fasting.
Liver damage usually accompanies skin or gastrointestinal GVHD. Even though liver GVHD may be suspected due to elevated transaminase levels, liver biopsy showing extensive bile duct damage with atypia and degeneration, as well as lymphocytic infiltration, is required to document its presence. Elevated serum bilirubin, alkaline phosphatase, and cholesterol levels are the earliest and most common signs, whereas coagulopathy and hyperammonemia are rare, but can develop in extremely severe cases. Some patients present with painful hepatomegaly, choluria, acholia, fluid retention, and pruritus.6,7 Stratification is based on serum bilirubin values: stage 1: 2-3mg/dl, stage 2: 3-6mg/dl, stage 3: 6-15mg/dl, stage 4:> 15mg/dl. Histologic findings consistent with liver GVHD are compromised bile ducts, epithelial dysmorphia, periportal and/or portal inflammation, and lobular necroinflammatory involvement, with or without pericellular or portal fibrosis.16
Another important factor to consider is patient comorbidity. There is an index for measuring complication risk, including death, in patients receiving HPCT that is based on the comorbidities the patients have before transplantation and is directly proportional to complication appearance.14,17 Therefore, we can predict which patients will require more intensive care, a situation especially true for patients with previous comorbidities. Such was the case with our patient #12, who had benefitted from greater conditioning, but the risk for invasive infection had risen considerably. Therefore, more studies are needed in the near future that can indicate the guidelines for establishing individualized treatment for each patient based on his or her clinical conditions prior to HPCT.
Ours is the first study on GVHD in post-HPCT pediatric patients conducted in Latin America. HPCT is not frequently performed worldwide, and even less so in Mexico. Given the small number of patients in the present study, its results cannot be generalized, and more studies need to be carried out. However, our study demonstrates that GVHD is indeed present in our population and its frequency will increase as more HPCTs are performed.
HPCT is a procedure that is rarely performed in the Mexican medical setting, and even though the liver and gastrointestinal GVHD frequency in our study was lower than that reported in other case series, these diseases will increasingly manifest in our environment. Risk factors for our population must still be determined, factors that will potentially aid in predicting GVHD. It is essential for each patient that undergoes HPCT to be evaluated by a gastroenterologist to determine the possibility of pre-existing gastrointestinal tract or liver damage prior to transplantation to determine which patients will most likely present with complications during the early and late recovery processes after HPCT. Thus, the aggressiveness with which immunosuppression is applied, and the care with which complications after transplantation must be managed, can be guided, whether in relation to previous comorbidity or to the complications characteristic of GVHD or HPCT. More studies whose aim is to identify both risk factors and protective factors for the development of liver and/or gastrointestinal GVHD must be conducted.
Ethical disclosuresProtection of human and animal subjects.The authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of data.The authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consent.The authors declare that no patient data appear in this article.
Financial disclosureNo financial support was received in relation to this study/article.
Conflict of interestThe authors declare that there is no conflict of interest.
Please cite this article as: Jaramillo-Esparza CM, Consuelo-Sánchez A, Acosta-Rodríguez-Bueno CP, Ramón-García G, Sadowinski-Pine SW, Escobar-Sánchez MA, et al. Enfermedad injerto contra huésped gastrointestinal y hepático en pacientes pediátricos con trasplante de células progenitoras hematopoyéticas en el Hospital Infantil de México Federico Gómez. Revista de Gastroenterología de México. 2018;83:385–392.