The approach to HCV infection begins with the directed search for risk factors linked to its acquisition. Therefore, our primary aim was to identify the prevalence of risk factors associated with HCV infections in insured individuals seen at the Hidalgo delegation of the IMSS.
Materials and methodsAn observational, descriptive, cross-sectional study was conducted through validated surveys that identified major and minor risk factors. In cases of major risk factors, the Advanced Quality™ RAPID-ANTI-HCV TEST Accutrack® tests were applied to detect anti-HCV. Patients with positive tests were referred to the Hepatology service for the diagnostic-therapeutic approach. Statistical analysis was performed through measures of central tendency and percentages.
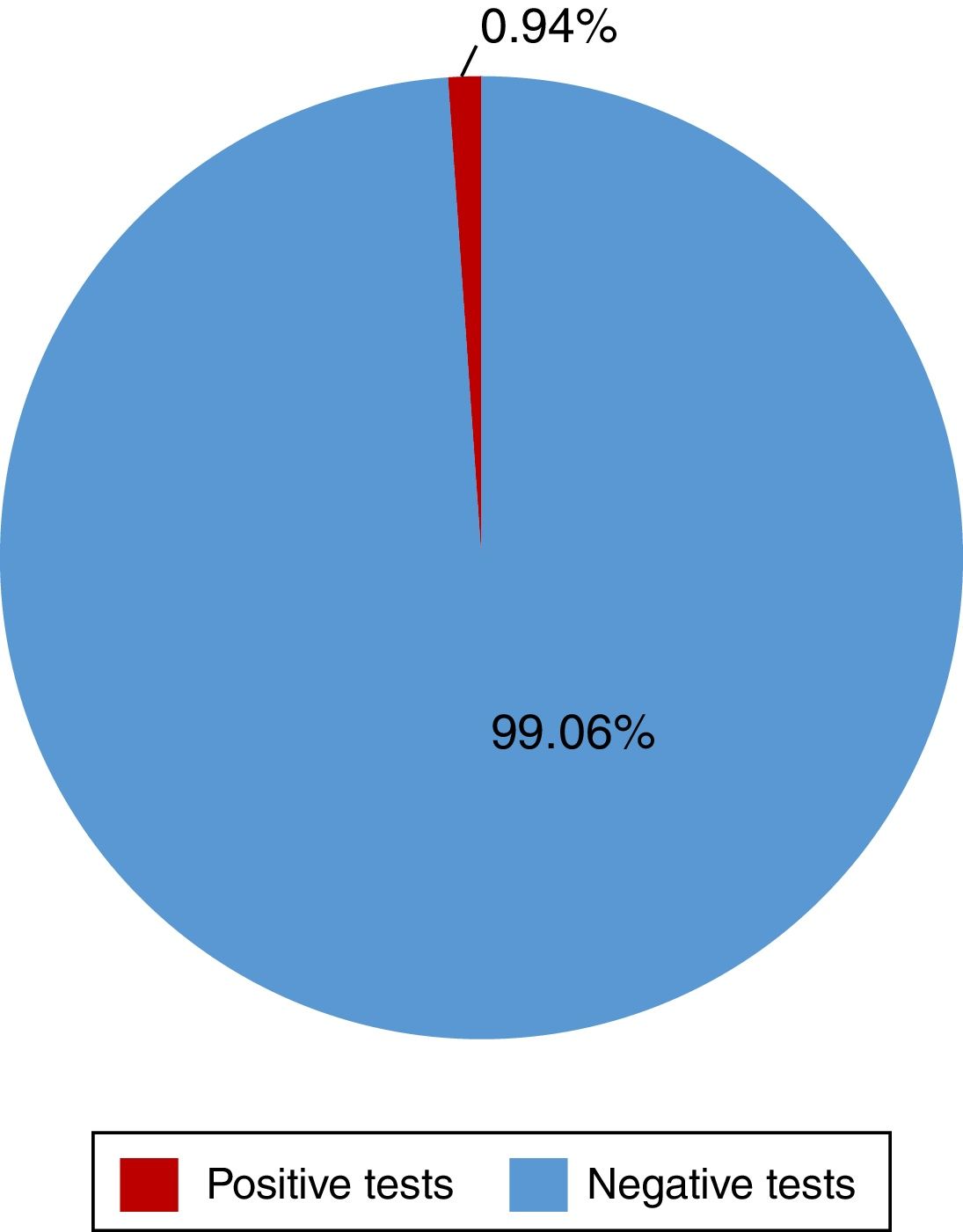
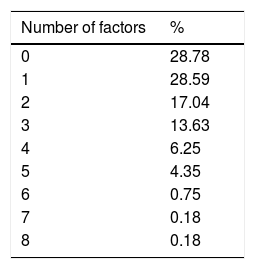
ResultsA total of 528 insured individuals were surveyed (95%CI with a 5% margin of error). Two hundred eighty-two rapid tests were performed. Five of them were positive (0.94%) and belonged to the patients in the dialysis/hemodialysis group. A total of 71.2% persons had positive risk factors. The association of 2 or more factors varied from 2 to 8 factors present at the same time. Of the entire study population, 6.25% presented with 4 risk factors and 4.35% presented with 5 risk factors at the same time.
ConclusionsNearly three quarters of the individuals surveyed were exposed to HCV acquisition. The association of 2 or more risk factors in patients demonstrated their collective potentiality for acquiring HCV. We identified persons receiving treatment with dialysis/hemodialysis and those with high-risk sexual practices as vulnerable groups for HCV infection and suggest that they receive promotion and prevention activities, as well as public policy management.
El abordaje de la infección por VHC inicia con la búsqueda intencionada de factores de riesgo vinculados a su adquisición. Así, nuestro objetivo principal fue identificar la prevalencia de factores de riesgo asociados a la infección por VHC en derechohabientes del IMSS, Delegación Hidalgo.
Material y métodosEstudio transversal, observacional, descriptivo, mediante encuestas validadas que identificaron factores de riesgo mayores y menores. En casos con factores de riesgo mayores se aplicaron pruebas rápidas Advanced Quality™ RAPID ANTI-VCH TEST accutrack® para detección de anti-HCV; ante una prueba reactiva se envió al servicio de Hepatología para abordaje diagnóstico-terapéutico. Se analizó estadísticamente con medidas de tendencia central y porcentajes.
ResultadosSe encuestó a un total de 528 derechohabientes (IC95% con 5% margen de error). Se realizaron 282 pruebas rápidas, 5 resultaron positivas (0.94%), pertenecientes al grupo de diálisis/hemodiálisis. El 71.2% tuvo factores de riesgo positivos. La asociación de dos o más factores varió entre 2 y hasta 8 factores presentados a la vez, siendo el 6.25 y el 4.35% del total de la población quienes presentaron 4 y hasta 5 factores a la vez, respectivamente.
ConclusionesCerca de 3/4 partes de los encuestados están expuestos a la adquisición del VHC. La asociación de 2 o más factores de riesgo en las personas evidenció la potencialidad colectiva de estos para adquirir VHC. Identificamos como grupos vulnerables personas en tratamiento con diálisis/hemodiálisis y prácticas sexuales de alto riesgo, en quienes sugerimos enfocar las actividades de promoción y prevención, así como la gestión de políticas públicas.
Approximately 180 million persons worldwide are infected with the hepatitis C virus (HCV), and at least one-third of that total will develop cirrhosis of the liver and hepatocellular carcinoma. HCV also significantly contributes to the prevalence of chronic hepatitis.1,2 The Fundación Mexicana de Salud Hepática reported a different prevalence of hepatitis C in the northern (2.0%), central (1.1%), and southern (1.5%) states of Mexico. Hepatitis C is primarily detected through the examination of risk factors associated with exposure to the virus. However, taking serologic samples, followed by RNA testing, is essential in individuals that belong to high-prevalence populations with a history of risk for exposure to determine the presence of the virus.1–3 The primary aim of our study was to identify the prevalence of risk factors associated with HCV infection in persons insured by the IMSS (Hidalgo sector).
Materials and methodsA descriptive, observational, prospective, cross-sectional study was conducted within the time frame of January to August 2015, in which a total of 528 individuals insured by the Instituto Mexicano del Seguro Social (IMSS), assigned to the Family Medicine Unit Zone Number 1 in the State of Hidalgo, were surveyed. The previously validated questionnaire consisted of 16 dichotomous items and it was the product of the combination of the following three questionnaires: The Rapid Test Campaign for Hepatitis C Detection Questionnaire, the Roche Risk Factors Questionnaire, and the Merck Sharp & Dohme Corporation Risk Factors for Hepatitis C Questionnaire, the use and application of which were all approved. The study questionnaire was divided into 2 sections: 6 items corresponded to major risk factors and 10 items corresponded to minor risk factors (annex A). Individuals of both sexes, ranging from 18 to 65 years of age, gave their informed consent to participate and were included in the study. Persons with a different cause of liver disease, those with a previous diagnosis of cirrhosis or chronic hepatopathy, persons under 18 years of age, and those with a primary noncommunicable chronic degenerative disease were excluded. An accutrack® Advanced Quality™ RAPID ANTI-HCV TEST for detecting antibodies to HCV was applied to all cases that presented with at least one major risk factor. If it was negative, the patient's participation in the study was concluded. If it was positive, the patient was immediately referred to the Hepatology Service to accelerate diagnosis and treatment. The cases with minor risk factors were referred to the Hepatology Service for comprehensive and specific care. Finally, the statistical analysis was carried out using measures of central tendency and percentages. The study, as required, was reviewed and approved by the ethics committee of the hospital.
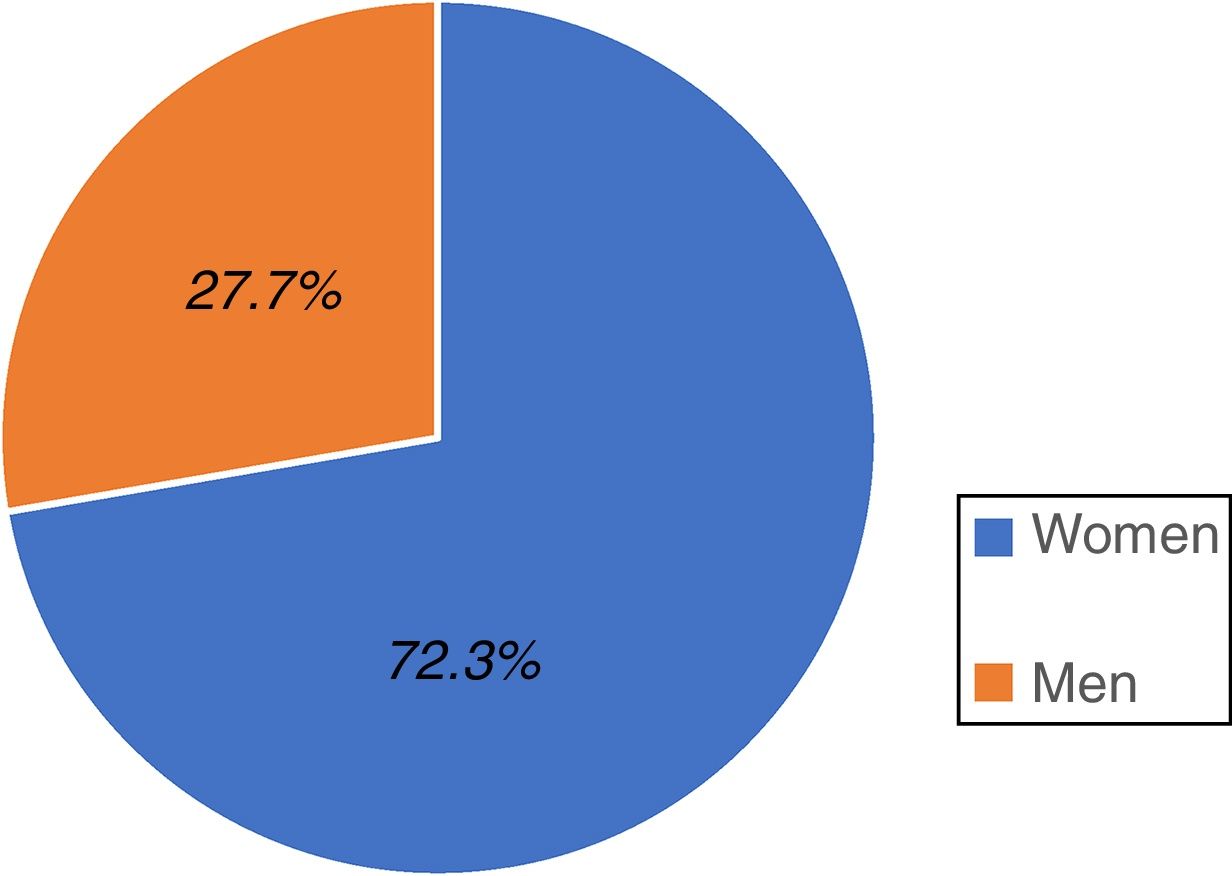
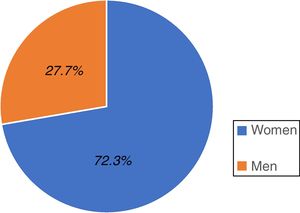
ResultsA total of 528 individuals insured by the IMSS (Hidalgo sector) participated in the study; 382 were women, representing 72.3% of the total population and 146 were men, accounting for 27.7% (fig. 1).
The age range of the total study population was 18 to 65 years, with a median of 37 years and a mode of 22 years. The prevalent age range of the total study population was 18 to 65 years.
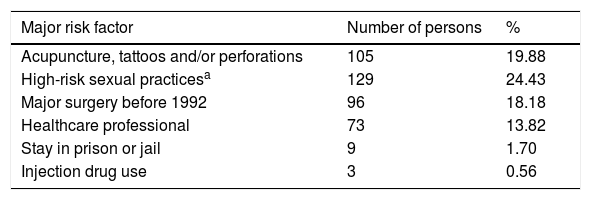
The most prevalent major risk factor of our study was «High-risk sexual practices », present in 129 of the insured individuals, equivalent to 24.43% of the total population, and the least prevalent major risk factor was «Injection drug use», present in 3 of the individuals, corresponding to 0.56% of the total population. The most prevalent minor risk factor was «Persons born between 1946 and 1965», found in 126 (23.86%) of the individuals of the total population. The least prevalent minor risk factor was «Mother carrying virus during pregnancy», of which there was one case, accounting for 0.18% of the total population (Table 1).
Prevalence of the major and minor risk factors for acquiring HCV in the total population studied.
| Major risk factor | Number of persons | % |
|---|---|---|
| Acupuncture, tattoos and/or perforations | 105 | 19.88 |
| High-risk sexual practicesa | 129 | 24.43 |
| Major surgery before 1992 | 96 | 18.18 |
| Healthcare professional | 73 | 13.82 |
| Stay in prison or jail | 9 | 1.70 |
| Injection drug use | 3 | 0.56 |
| Minor risk factor | Number of persons | % |
|---|---|---|
| Persons born between 1946 and 1965 | 126 | 23.86 |
| Used-needle puncture | 103 | 19.50 |
| Direct family history of hepatitis C | 66 | 12.50 |
| History of transfusions | 37 | 7.00 |
| Previous diagnosis of hepatitis C | 14 | 2.65 |
| Inhaled drug use | 9 | 1.70 |
| Hemophilia | 9 | 1.70 |
| Sexual partner diagnosed with viral hepatitis | 6 | |
| Dialysis | 53 | 10.03 |
| Mother carrying virus during pregnancy | 1 | 0.18 |
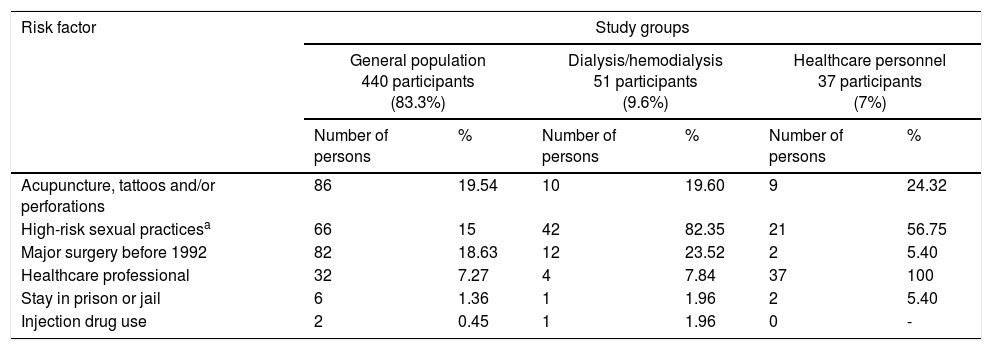
The total population was classified into 3 groups: 440 persons belonging to the general population, representing 83.3% of the total population; 51 persons with chronic kidney disease in replacement therapy with peritoneal dialysis/hemodialysis, representing 9.6% of the total population; and 37 persons belonging to the healthcare personnel, representing 7% of the total population. Of the general population group, the most prevalent major risk factor was «Acupuncture, tattoos and/or perforations», present in 86 persons, corresponding to 19.54% of that study group, and the most prevalent minor risk factor was «Persons born between 1946 and 1965», present in 102 (23.18%) individuals of that study group. Of the dialysis/hemodialysis group, the most prevalent major risk factor was «Risky sexual practices», present in 42 persons, corresponding to 82.35% of that study group, and the most prevalent minor risk factor was «Persons born between 1946 and 1965», present in 20 (39.21%) individuals of that study group. And finally, of the healthcare personnel group, the most prevalent major risk factor was «High-risk sexual practices», present in 21 persons, corresponding to 76.55% of that study group, and the most prevalent minor risk factor was «Used-needle puncture», present in 16 (43.24%) persons of that study group (Table 2).
Prevalence of major risk factors for acquiring HCV by study group.
| Risk factor | Study groups | |||||
|---|---|---|---|---|---|---|
| General population 440 participants (83.3%) | Dialysis/hemodialysis 51 participants (9.6%) | Healthcare personnel 37 participants (7%) | ||||
| Number of persons | % | Number of persons | % | Number of persons | % | |
| Acupuncture, tattoos and/or perforations | 86 | 19.54 | 10 | 19.60 | 9 | 24.32 |
| High-risk sexual practicesa | 66 | 15 | 42 | 82.35 | 21 | 56.75 |
| Major surgery before 1992 | 82 | 18.63 | 12 | 23.52 | 2 | 5.40 |
| Healthcare professional | 32 | 7.27 | 4 | 7.84 | 37 | 100 |
| Stay in prison or jail | 6 | 1.36 | 1 | 1.96 | 2 | 5.40 |
| Injection drug use | 2 | 0.45 | 1 | 1.96 | 0 | - |
| Minor risk factor | Study groups | |||||
|---|---|---|---|---|---|---|
| General population 440 participants (83.3%) | Dialysis/hemodialysis 51 participants (9.6%) | Healthcare personnel 37 participants (7%) | ||||
| Number of persons | % | Number of persons | % | Number of persons | % | |
| Persons born between 1946 and 1965 | 102 | 23.18 | 20 | 39.21 | 4 | 10.81 |
| Used-needle puncture | 78 | 17.72 | 9 | 17.64 | 16 | 43.24 |
| Direct Family history of hepatitis C | 51 | 11.59 | 11 | 21.56 | 4 | 10.81 |
| History of transfusions | 25 | 5.68 | 11 | 21.56 | 1 | 2.70 |
| Previous diagnosis of hepatitis C | 12 | 2.72 | 2 | 3.92 | 0 | - |
| Inhaled drug use | 8 | 1.81 | 0 | — | 1 | 2.70 |
| Hemophilia | 8 | 1.81 | 1 | 1.96 | 0 | - |
| Sexual partner diagnosed with viral hepatitis | 5 | 1.13 | 1 | 1.96 | 0 | - |
| Dialysis | 3 | 0.68 | 51 | 100 | 0 | - |
| Mother carrying virus during pregnancy | 1 | 0.22 | 0 | — | 0 | - |
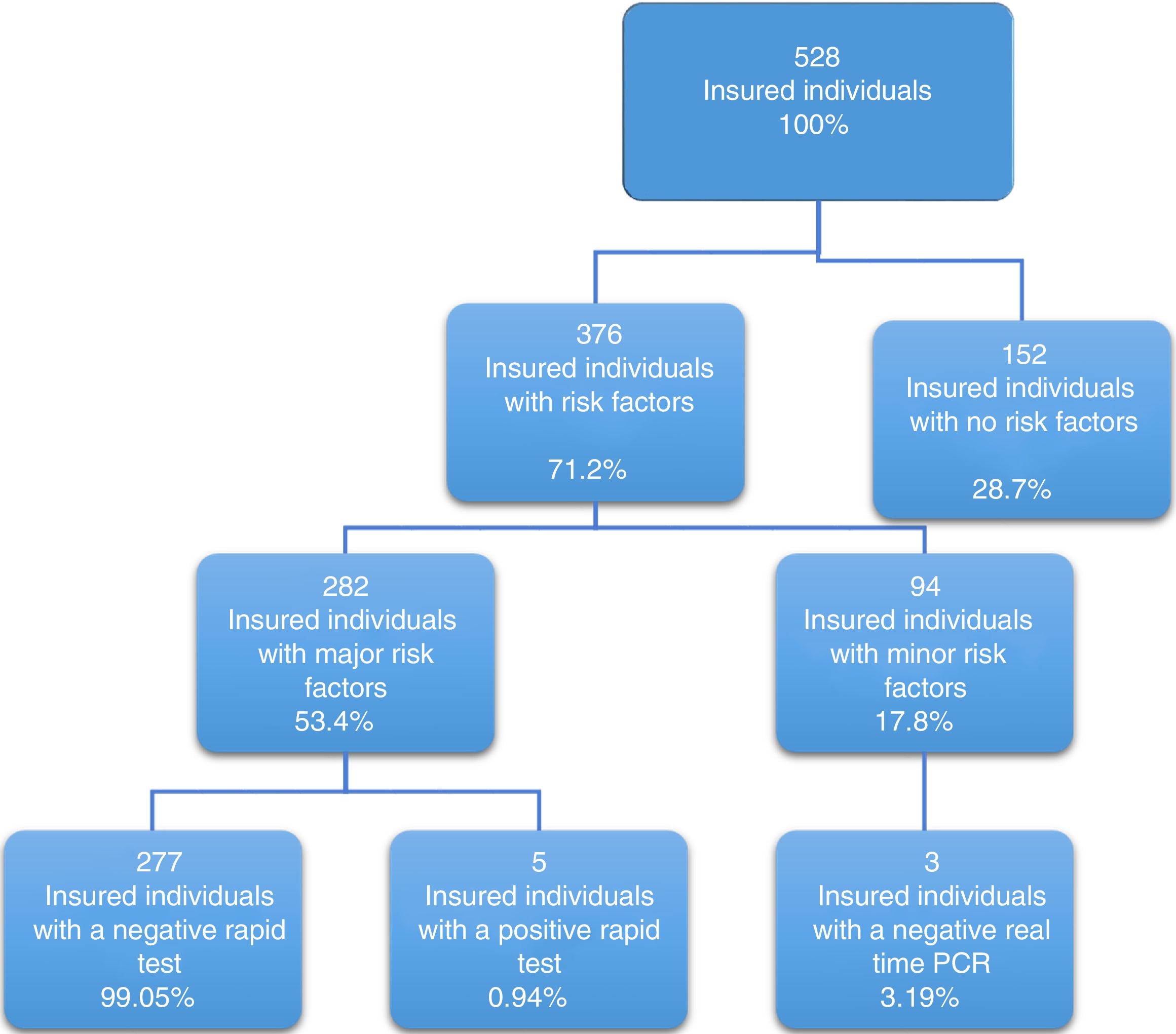
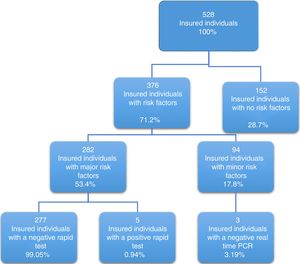
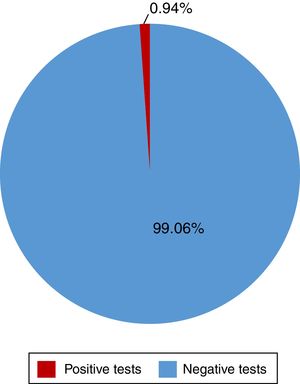
Of the 528 insured individuals that answered the questionnaire, 152 did not present with positive risk factors, corresponding to 28.7% of the total population, whereas 376 presented with at least one positive risk factor, representing 71.2% of the total population. Of those individuals, 94 (17.8%) had minor risk factors and 282 (53.4%) had major risk factors. The persons with major risk factors were screened through the rapid blood test, and the results were negative in 277 (99.05%) of them. Finally, 5 insured individuals, corresponding to 0.94% of the total population, had positive results in the rapid test for detecting hepatitis C virus infection, all of whom belonged to the peritoneal dialysis/hemodialysis group (figs. 2 and 3).
The association of two or more risk factors in a person varied from 2 to 8 risk factors at the same time. A total of 6.25% of the total population simultaneously presented with 4 risk factors and 4.35% presented with 5 (Table 3).
Of the individuals that had a positive rapid test, only 2 had the single risk factor of receiving kidney function replacement therapy through peritoneal dialysis or hemodialysis. The rest of the persons had the simultaneous presentation of at least 3 and up to 5 positive risk factors that included high-risk sexual practices, major surgery before 1992, and a history of having had tattoos, undergoing acupuncture, or having perforations.
DiscussionHCV infection is currently a global public health problem. Approximately 2.8% of the population of the world is infected. The regions with greater prevalence are eastern and central Asia (3.8 and 3.7%, respectively) and Africa/the Middle East (3.5%). Central America has an estimated prevalence of 1.6%, whereas Mexico has a reported prevalence of 1.4%.1–8 Only 14 cases in the State of Hidalgo were reported by the SINAVE up to the last week of 2016.9
HCV infection is acquired through direct contact with biologic fluids and/or contaminated surgical material, the latter being more frequent in Mexico. Sexual transmission of the disease is uncommon in Mexico, but the risk for intravenous contagion is greater, being prevalent in the northern states, where injection drug use is frequent.10,11 However, high-risk sexual practices have been identified as a special subgroup: sexual intercourse between men, engaging in sex with male or female sex workers, and sexual promiscuity (2 or more sexual partners within a 6-month period) appear to be on the rise in relation to HCV infection.1,2,11–14 The group of persons born between 1945 and 1964, known as the «baby boomers», is considered a risk group, due to reports of its greater injection drug use, polygamous sexual activity, and blood transfusions before 1992. Likewise, virus acquisition has been identified in persons that undergo some kind of aesthetic procedure, such as tattoos and/or piercings, persons that have been in prison or jail, as well as vertical infection transmission.1,2,10,15–20 Healthcare personnel is another group identified to be more susceptible to acquiring HCV infection than the rest of the population.1,2,5,6,8,21
Despite the Mexican healthcare regulations with respect to the disposal of blood, in place since 1993, persons that are in contact with said fluid and persons that receive kidney function replacement therapy through dialysis or hemodialysis have been identified as at-risk populations.1,2,22–24
Burguete-García et al.25 found seroprevalence of 1.5% in Mexico in a study conducted from 2006 to 2009. Half of the study subjects had chronic HCV infection. Similar results were reported by Valdespino et al.,26 who, in 2005, studied serums with antibodies to HCV and with RNA, based on data from a 2000 national health survey. They found seroprevalence of 1.4% in Mexico, with chronic infection making up the fourth part of that percentage. The results of both of those studies contrasted with ours. An influencing factor in that regard was the use of different screening and confirmation tests. We used the Advanced Quality™ RAPID ANTI-HCV tests distributed by accutrack® and manufactured by InTecProducts, Inc (Xiamen, China), and real time PCR to confirm active chronic infection. Burguete-García et al.25 used the ELISA (Johnson and Johnson Company) method for measuring antibodies, confirming chronic infection through the COBAS AMPLICOR RT-PCR test and Valdespino et al.26 used the ELISA microparticle assays for anti-HCV IgG antibodies version 3.0 (Axsym, Abbott Laboratories, USA) and the RT-PCR test for determining HCV RNA (Roche Molecular Diagnostic, USA), both of which are tests with greater sensitivity and specificity.
On the other hand, by comparing studies that used the same rapid tests that we did for detecting antibodies to HCV, our results were similar to those of Mena-Quintero and Molina-Cornelio, 27 but different from those reported by Castañeda-Huerta et al.28 and López-Colombo et al.,29 who applied rapid tests in an open population and in insured individuals and their relatives seen at primary care units. They reported seroreactive subjects detected by those tests in 4 and 1.17%, respectively. Seroreactivity was confirmed in 31% of those individuals through viral RNA testing.
The most prevalent risk factors in our study were: tattoos, piercings and/or acupuncture, high-risk sexual practices, major surgery before 1992, birth between 1946 and 1965, and accidental puncture with used needles. Our results were similar to those of other studies, except for the scant use of injection drugs. Other authors have reported that activity at much higher frequencies, considering it one of the most relevant risk factors for acquiring the virus. In our population, only two persons stated they used injection drugs, and neither of them used inhaled drugs, which are known to potentiate the risk for acquisition of the virus.25–30
The most prevalent risk factor in the individuals in our study that had positive rapid tests (60%, 3 persons) was high-risk sexual practices, in contrast to that reported by Mena-Quintero and Molina-Cornelio.27 They identified surgery before 1995 as the main risk factor, followed by the use of potentially contaminated needles (41%), and in third place, high-risk sexual practices (31%). Two of their subjects (40%) had no risk factors other than having received dialysis/hemodialysis, which was similar to that reported by Castañeda-Huerta et al.28 They found 2 persons (9.5%) with a positive rapid test and no risk factor associated with the development of the disease. A greater frequency of blood transfusions was found by other authors, compared with our results.28–30
There were no risk factors associated with the development of hepatitis C in 28.7% of our population, showing null prevalence in the patients born between 1946 and 1965. The importance of that factor alludes to the post-war liberal sociocultural movements and poor sanitary practices for the control of infections transmitted through blood that occurred in North America and England, and to a lesser degree in Mexico. In contrast, Castañeda-Huerta et al.28 found that 11 subjects born between 1945-1965 were carriers of the virus, corresponding to 52% of their sample.
With respect to the association of positive risk factors (found in 71.2% of the total population surveyed), one-third of those individuals had only one positive risk factor, whereas 3.57, 3.15, and 0.84% had 4, 5, and 6 simultaneous positive factors, respectively. The finding that a large percentage of the population was exposed to at least one risk factor, despite having obtained 277 negative anti-HCV tests, was striking. Thus, the importance of determining multiple risk factors associated with the acquisition of the disease in a Mexican state with a relatively low percentage of hepatitis C, and the relevance of duplicating the study in other states of the republic with a higher number of patients diagnosed with the disease.
There is no statistical information provided by other analyses with which to compare data, making our study the first to pave the way for the epidemiologic evaluation of HCV in the State of Hidalgo. Another advantage of our analysis was the fact that we were able to carry out a rapid test on all patients in the study population that had at least one major risk factor for the disease.
All patients with at least one minor risk factor should have had a rapid blood test to prevent their loss from the study, given that only 3.14% of those patients kept their appointments to undergo real time PCR. A population with greater statistical significance in the peritoneal dialysis/hemodialysis subgroup should also have been included in our study to prevent bias in the results, even though the total study population had a 95% confidence interval and a 5% sampling error. We only included patients covered by the medical insurance of the IMSS in our analysis, but it would be prudent to replicate the study on an open population, regardless of insurance coverage, to avoid group bias.
ConclusionsBased on our results, we can conclude that over half of the population surveyed was exposed to HCV. The collective potentiality of the risk factors a person has for acquiring and developing the infection should be studied. We identified persons that undergo kidney function replacement therapy and persons that engage in high-risk sexual practices as groups that were vulnerable for contracting HCV. Therefore, we suggest that health education and prevention activities and public policy management be directed at those individuals.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Financial disclosureNo financial support was received in relation to this study/article.
Conflict of interestThe authors declare that there is no conflict of interest.
The advanced quality accutrack®TMRAPID TEST ANTI-VCH tests were provided by the MSD S.A. de C. V. laboratories without their involvement in the application of the questionnaires, rapid tests, analyses, or any other aspect of the study.
Please cite this article as: Contreras-Omaña R, García-Lemus FJ, García-Camacho A. Factores de riesgo para adquirir VHC en una institución de salud en Hidalgo. Revista de Gastroenterología de México. 2019;84:36–43.