Self-expanding metallic stents (SEMSs) are ideal treatment for malignant gastric outlet obstruction (MGOO) in patients with a short life expectancy, but stent dysfunction is frequent. The primary aim of our study was to identify the predictive factors of SEMS dysfunction in MGOO and the secondary aim was to determine the technical success, clinical success, and nutritional impact after SEMS placement.
Materials and methodsA retrospective, longitudinal study was conducted at the gastrointestinal endoscopy department of the Instituto Nacional de Cancerología in Mexico City. Patients diagnosed with MGOO that underwent SEMS placement within the time frame of January 2015 to May 2018 were included. We utilized the gastric outlet obstruction scoring system to determine clinical success and SEMS dysfunction.
ResultsThe study included 43 patients, technical success was 97.7% (n = 42), and clinical success was 88.3% (n = 38). SEMS dysfunction presented in 30.2% (n = 13) of the patients, occurring in < 6 months after placement in 53.8% (n = 7) of them. In the univariate analysis, the histologic subtype, diffuse gastric adenocarcinoma, (p = 0.02) and uncovered SEMS (p = 0.02) were the variables associated with dysfunction. Albumin levels and body mass index did not increase after SEMS placement. Medical follow-up was a mean 5.8 months (1-24 months).
ConclusionsSEMS demonstrated adequate technical and clinical efficacy in the treatment of MGOO. SEMS dysfunction was frequent and diffuse-type gastric cancer and uncovered SEMS appeared to be dysfunction predictors.
Los stents metálicos autoexpandibles (SEMS) son el tratamiento ideal de la obstrucción de salida gástrica maligna (OSGM) en pacientes con expectativa de vida corta, sin embargo, su disfunción es frecuente. El objetivo principal fue conocer los factores predictores de disfunción del SEMS en OSGM y los objetivos secundarios conocer éxito técnico, éxito clínico e impacto nutricional posterior al SEMS.
Material y métodosEstudio longitudinal, retrospectivo en el departamento de endoscopia gastrointestinal del Instituto Nacional de Cancerología de la Ciudad de México. Se incluyeron pacientes con diagnóstico de OSGM con colocación de SEMS de enero 2015 a mayo 2018. Utilizamos el sistema de obstrucción de salida gástrica (GOOSS) para establecer éxito clínico y disfunción del SEMS.
ResultadosIncluimos 43 pacientes, el éxito técnico fue 97.7% (n = 42), éxito clínico 88.3% (n = 38). La disfunción del SEMS se presentó en el 30.2% (n = 13), de estos pacientes en el 53.8% (n = 7) la disfunción ocurrió en < 6 meses posterior a su colocación. En el análisis univariado el subtipo histológico adenocarcinoma gástrico difuso (p = 0.02) y SEMS no cubierto (SEMS-NC) (p = 0.02) fueron las variables asociadas a disfunción. La albumina e índice de masa corporal (IMC) no aumentaron posterior al SEMS. El seguimiento medio fue 5.8 meses (1-24 meses).
ConclusionesLos SEMS tienen adecuada eficacia técnica y clínica en el tratamiento de la OSGM. La disfunción del SEMS es frecuente y el tipo histológico cáncer gástrico difuso y SEMS-NC parecen ser predictores de disfunción.
Gastric outlet obstruction (GOO) is the complete or incomplete obstruction of the distal stomach, pylorus, or proximal duodenum caused by an obstructive lesion or extrinsic compression. The most frequent causes of malignant gastric outlet obstruction (MGOO) are pancreatic cancer and gastric cancer, with an incidence of 15-25%.1–4
Gastrojejunal anastomosis (GJA) was previously the standard treatment for GOO. However, the endoscopic placement of a self-expanding metallic stent (SEMS) enables faster resumption of oral intake and a shorter hospital stay.5,6 Thus, SEMSs have become ideal treatment in patients with a short life expectancy. Numerous studies have confirmed their efficacy and safety,7–12 with clinical success of 77% to 94%.12–25 Nevertheless, SEMS dysfunction presents in up to 26% of patients,26 causing frequent endoscopic re-interventions and increasing costs and morbidity. Few studies have been conducted on the factors that predict SEMS dysfunction in MGOO, such as a Karnofsky score (KS) < 50,26,27 carcinomatosis,28 ascites,28,29 and SEMS expansion < 30%,27 and their results have not been replicated in other similar studies.
The primary aim of the present study was to know the predictive factors of SEMS dysfunction in patients with MGOO. The secondary aims were to determine the technical success, clinical success, and nutritional impact after SEMS placement.
Materials and methodsA retrospective, observational, longitudinal, analytic study was conducted at the Department of Gastrointestinal Endoscopy of the Instituto Nacional de Cancerología in Mexico City, within the time frame of January 2015 to May 2018.
PatientsPatients diagnosed with MGOO that presented with unresectable disease or were in palliative treatment, and that underwent SEMS placement, were included in the study.
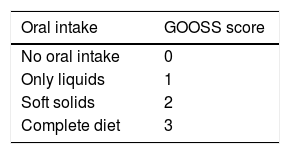
The inclusion criteria were patients above 18 years of age, with endoscopically demonstrated obstruction of the distal stomach or duodenum that made oral intake difficult, patients that had a gastric outlet obstruction scoring system (GOOSS) score ≤ 2 (Table 1), biopsies conclusive of malignant neoplasia, and computed axial tomography studies from which the presence of ascites, carcinomatosis, distant metastasis, and unresectable disease was evaluated. The exclusion criteria were pregnant patients, gastrointestinal perforation, peritonitis, obstruction distal to the MGOO, SEMS placement not performed at the Instituto Nacional de Cancerología, no electronic case record, and follow-up that was under 7 days.
TechniqueAn Olympus GIF-1T140 and GIF-1TQ160 therapeutic gastroscope was utilized for SEMS placement. The location and length of the obstruction was evaluated by endoscopy and fluoroscopy with contrast medium. A biliary hydrophilic guidewire was then introduced through the stricture and the SEMS was introduced over the guidewire and released under endoscopic and fluoroscopic guidance. The selection of the length of the SEMS was based on the length of the stricture. An additional 4 cm was considered to ensure 2 cm of stent before and after the ends of the stricture. SEMS selection was based on the experience of the endoscopist. Four endoscopists with more than 10 years of experience in SEMS placement participated in the study. The 3 types of SEMS utilized were the WallFlex Duodenal Stent (Boston Scientific) and the WALLSTENT Duodenal Stent (Boston Scientific), both uncovered stents (ucSEMSs), and the Niti-S Pyloric/Duodenal Stent (Taewoong Medical), a partially covered SEMS (pcSEMS).
Measurement and definition of the variablesWe used the GOOSS, which assigns points depending on the patient’s level of oral intake, enabling oral intake capacity to be objectively evaluated (Table 1). The GOOSS was created in 2002 as a scoring system to assess oral intake in patients with malignant dysphagia30 and is currently the scale used in numerous studies on MGOO.26,27,29
Clinical success was defined as a GOOSS score ≥ 2 after 7 days of SEMS placement. Technical success was defined as the precise positioning of the SEMS at the obstruction site. SEMS dysfunction was defined as the reappearance of symptoms of GOO with a GOOSS score < 2 and radiologic or endoscopic evidence of tumor ingrowth or SEMS collapse, breakage, migration, or food impaction. The duration of SEMS permeability was defined as the time from SEMS placement to its dysfunction. When there was no dysfunction, SEMS permeability duration was considered the same as the length of time of the patient’s subsequent survival.
The following variables for predicting SEMS dysfunction were analyzed: age, sex, primary cancer organ, histologic type, obstruction location (antrum, pylorus, duodenum), stricture length, obstruction type (intrinsic or extrinsic), KS, clinical stage, ascites, carcinomatosis, SEMS type (commercial brand, pcSEMS, ucSEMS), and adjuvant treatment (chemotherapy and radiotherapy after SEMS placement).
Intrinsic stricture was considered when the endoscopically viewed tissue had a tumor-like aspect that conditioned a reduction in the gastric or duodenal lumen, and extrinsic stricture was considered when there was a reduction in the gastric or duodenal lumen with normal mucosa.
To evaluate the nutritional impact of SEMS in MGOO, body mass index (BMI) and serum albumin levels were assessed before and after SEMS placement.
Follow-upFollow-up was carried out through the electronic records up to May 2018, death of the patient, or loss to follow-up.
Statistical analysisUtilizing the SPSS version 23 program, a descriptive analysis was performed with measures of dispersion for the quantitative variables and proportions for the qualitative variables. A univariate model was used for the inferential analysis, employing the Student’s t test for the quantitative variables and the chi-square test for the qualitative variables. Statistical significance was set at a p < 0.05, with a 95% confidence interval (95% CI).
Ethical disclosuresThe authors declare that no experiments were conducted on humans or animals in the present study. Written statements of informed consent were obtained from all the patients, with absolute maintenance of their anonymity. The present study was approved by the ethics committee of the Instituto Nacional de Cancerología in Mexico City.
ResultsForty-five patients were evaluated. Two patients were excluded from the study, one due to a follow-up period under 7 days and one due to a lack of information in the electronic case record. A total of 43 patients with MGOO diagnosis and SEMS placement were included in the study.
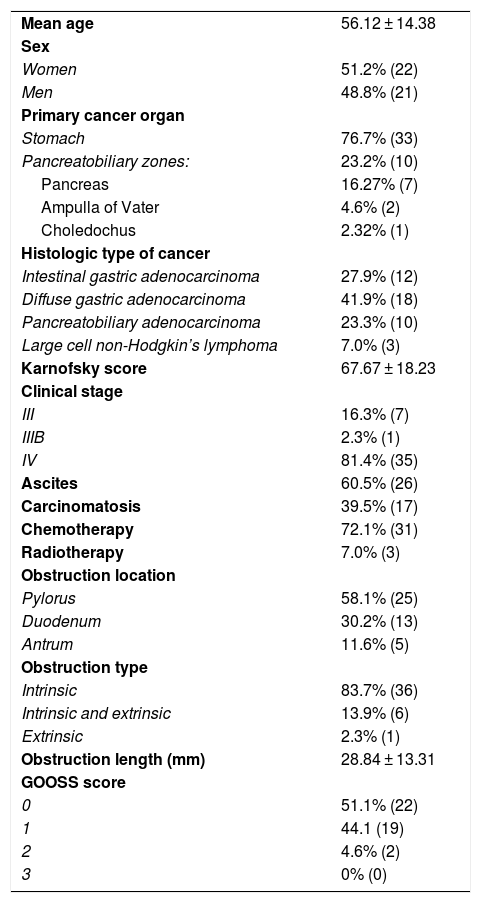
Patient characteristicsTable 2 shows the demographic and clinical characteristics of the study patients. A total of 51.2% (n = 22) of the patients were women and 48.8% (n = 21) were men, and the mean patient age was 56.12 ± 14.38 years. The stomach was the primary cancer organ in 76.7% (n = 33) of the patients and primary involvement was pancreatobiliary in 23.2% (n = 10) (pancreas in 7, ampulla of Vater in 2, common bile duct in 1). The histologic type of cancer was diffuse gastric adenocarcinoma in 41.9% (n = 18), intestinal gastric adenocarcinoma in 27.9% (n = 12), pancreatobiliary adenocarcinoma in 23.3% (n = 10), and large cell Hodgkin’s lymphoma in 7.0% (n = 3). The KS was 67.67 ± 18.23. A total of 16.3% of the patients (n = 7) had clinical stage III disease, 2.3% (n = 1) had stage IIIB, and 81.4% (n = 35) had stage IV. A total of 60.5% (n = 26) of the patients presented with ascites and 39.5% (n = 17) had carcinomatosis. The most frequent obstruction location was the pylorus, found in 58.1% (n = 25) of the patients, followed by the duodenum in 30.2% (n = 13) and the antrum in 11.6% (n = 5). There was intrinsic obstruction in 83.7% of the patients (n = 36), intrinsic and extrinsic obstruction in 13.9% (n = 6), and extrinsic obstruction in 2.3% (n = 1). The length of the obstruction was 28.84 ± 13.31 mm. The SEMS utilized were: WallFlex 72.1%, WALLSTENT 14.0%, and Niti-S 13.9%, of which 95.3% were ucSEMSs and 4.7% were pcSEMS. A total of 72.1% of the patients (n = 31) received adjuvant chemotherapy and 7.0% (n = 3) underwent adjuvant radiotherapy. Seven patients presented with bile duct obstruction. Six of them required endoscopic bile duct drainage (through endoscopic retrograde cholangiopancreatography or endoscopic ultrasound) and one patient had percutaneous bile duct drainage.
Patient demographic and clinical characteristics (n = 43).
| Mean age | 56.12 ± 14.38 |
| Sex | |
| Women | 51.2% (22) |
| Men | 48.8% (21) |
| Primary cancer organ | |
| Stomach | 76.7% (33) |
| Pancreatobiliary zones: | 23.2% (10) |
| Pancreas | 16.27% (7) |
| Ampulla of Vater | 4.6% (2) |
| Choledochus | 2.32% (1) |
| Histologic type of cancer | |
| Intestinal gastric adenocarcinoma | 27.9% (12) |
| Diffuse gastric adenocarcinoma | 41.9% (18) |
| Pancreatobiliary adenocarcinoma | 23.3% (10) |
| Large cell non-Hodgkin’s lymphoma | 7.0% (3) |
| Karnofsky score | 67.67 ± 18.23 |
| Clinical stage | |
| III | 16.3% (7) |
| IIIB | 2.3% (1) |
| IV | 81.4% (35) |
| Ascites | 60.5% (26) |
| Carcinomatosis | 39.5% (17) |
| Chemotherapy | 72.1% (31) |
| Radiotherapy | 7.0% (3) |
| Obstruction location | |
| Pylorus | 58.1% (25) |
| Duodenum | 30.2% (13) |
| Antrum | 11.6% (5) |
| Obstruction type | |
| Intrinsic | 83.7% (36) |
| Intrinsic and extrinsic | 13.9% (6) |
| Extrinsic | 2.3% (1) |
| Obstruction length (mm) | 28.84 ± 13.31 |
| GOOSS score | |
| 0 | 51.1% (22) |
| 1 | 44.1 (19) |
| 2 | 4.6% (2) |
| 3 | 0% (0) |
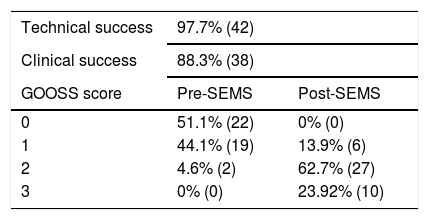
The technical success of SEMS placement was 97.7% (n = 42) and the clinical success was 88.3% (n = 38). Initial SEMS placement was technically unsuccessful in one patient with obstruction in the antrum because the SEMS was released in the duodenum, but a second SEMS was successfully placed at the obstruction site during the same endoscopy. A total of 95.3% (n = 41) of the patients had a GOOSS score of 0 or 1 prior to SEMS placement and 86% (n = 37) had a GOOSS score of 2 or 3 after SEMS placement (Table 3).
Self-expanding metallic stent dysfunctionSEMS dysfunction occurred in 30.2% (n = 13) of the patients, with the following characteristics: 38.5% (n = 5) were men and 61.5% (n = 8) were women; the stomach was the primary cancer organ in the majority of patients (92.3%) (n = 12); the most frequent histologic type was diffuse gastric adenocarcinoma (46.2%) (n = 6), followed by intestinal gastric adenocarcinoma (23.1%) (n = 3); the majority of the patients had clinical stage IV disease (92.3%) (n = 12), 76.9% (n = 10) presented with ascites, and 38.5% (n = 5) had carcinomatosis. The most frequent obstruction site was the pylorus (76.9%) (n = 10), followed by the duodenum (15.4%) (n = 2); 92% of the patients had intrinsic obstruction (n = 12) and 7.6% had intrinsic and extrinsic obstruction (n = 1); the WallFlex stent was the SEMS used in 69.2% (n = 9) of the patients and ucSEMSs were used in 84.6% (n = 11). The majority of patients received adjuvant chemotherapy (84.6%) (n = 11) and only 7.7% (n = 1) received adjuvant radiotherapy.
Tumor ingrowth was the cause of SEMS dysfunction (Table 4) in 92.3% (n = 12) of the patients, food impaction in 7.6% (n = 1), and lack of angled expansion in 7.6% (n = 1). One patient had two causes of SEMS dysfunction (tumor ingrowth and food impaction). SEMS dysfunction was resolved in 53.8% of the patients (n = 7). The resolution method was the placement of another SEMS in 3 patients, argon plasma electrocoagulation in 2 patients, and balloon dilation in 2 patients. In the unresolved SEMS dysfunction group, endoscopic resolution was not attempted in 2 patients and argon plasma coagulation failed in 3 patients, after which a nasojejunal tube was placed.
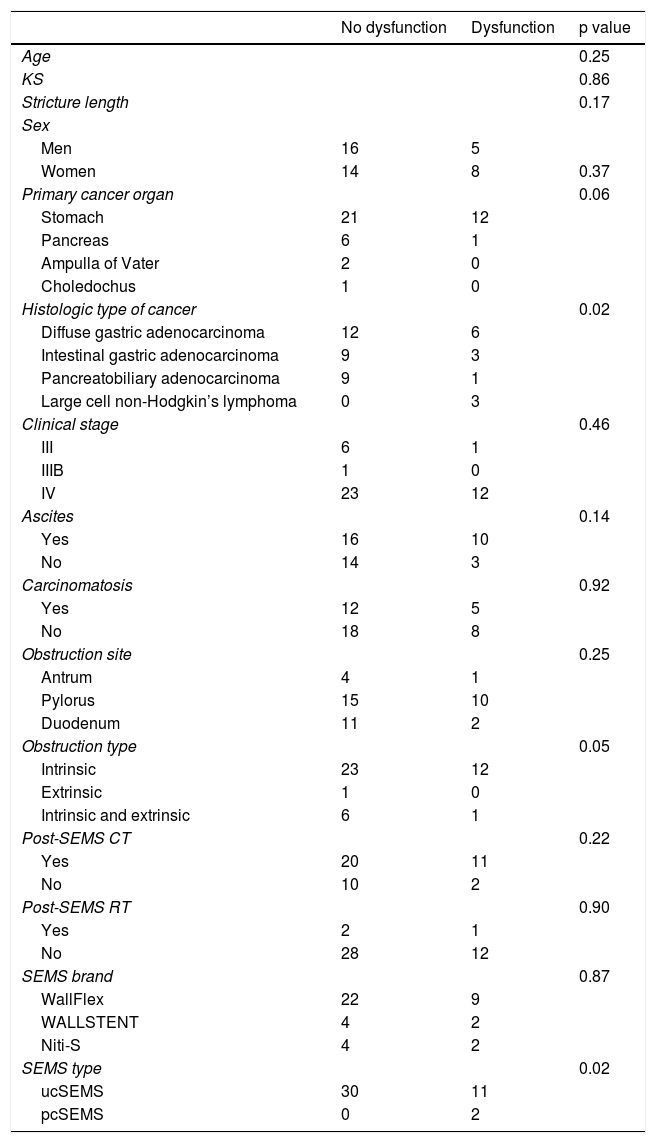
In the univariate analysis (Table 5), the histologic subtype of diffuse gastric adenocarcinoma (p = 0.02) and ucSEMSs (p = 0.02) were the variables associated with SEMS dysfunction that reached statistical significance. In addition, the stomach as the primary cancer organ (p = 0.06) and intrinsic obstruction (p = 0.57) showed a tendency to be significant predictors for SEMS dysfunction.
Univariate analysis of the factors associated with SEMS dysfunction.
| No dysfunction | Dysfunction | p value | |
|---|---|---|---|
| Age | 0.25 | ||
| KS | 0.86 | ||
| Stricture length | 0.17 | ||
| Sex | |||
| Men | 16 | 5 | |
| Women | 14 | 8 | 0.37 |
| Primary cancer organ | 0.06 | ||
| Stomach | 21 | 12 | |
| Pancreas | 6 | 1 | |
| Ampulla of Vater | 2 | 0 | |
| Choledochus | 1 | 0 | |
| Histologic type of cancer | 0.02 | ||
| Diffuse gastric adenocarcinoma | 12 | 6 | |
| Intestinal gastric adenocarcinoma | 9 | 3 | |
| Pancreatobiliary adenocarcinoma | 9 | 1 | |
| Large cell non-Hodgkin’s lymphoma | 0 | 3 | |
| Clinical stage | 0.46 | ||
| III | 6 | 1 | |
| IIIB | 1 | 0 | |
| IV | 23 | 12 | |
| Ascites | 0.14 | ||
| Yes | 16 | 10 | |
| No | 14 | 3 | |
| Carcinomatosis | 0.92 | ||
| Yes | 12 | 5 | |
| No | 18 | 8 | |
| Obstruction site | 0.25 | ||
| Antrum | 4 | 1 | |
| Pylorus | 15 | 10 | |
| Duodenum | 11 | 2 | |
| Obstruction type | 0.05 | ||
| Intrinsic | 23 | 12 | |
| Extrinsic | 1 | 0 | |
| Intrinsic and extrinsic | 6 | 1 | |
| Post-SEMS CT | 0.22 | ||
| Yes | 20 | 11 | |
| No | 10 | 2 | |
| Post-SEMS RT | 0.90 | ||
| Yes | 2 | 1 | |
| No | 28 | 12 | |
| SEMS brand | 0.87 | ||
| WallFlex | 22 | 9 | |
| WALLSTENT | 4 | 2 | |
| Niti-S | 4 | 2 | |
| SEMS type | 0.02 | ||
| ucSEMS | 30 | 11 | |
| pcSEMS | 0 | 2 | |
The SEMS permeability duration in the patients with SEMS dysfunction was 5.4 ± 3.5 months and dysfunction occurred in 7 patients in < 6 months after placement.
Nutritional impactBMI was 20.6 ± 3.2 kg/m2 prior to SEMS placement and 20.7 ± 3.3 kg/m2 after SEMS placement. The serum albumin level was 3.23 ± 0.62 g/dl before SEMS placement and 3.05 ± 0.64 g/dl after SEMS placement. A sub-analysis was carried out on the 28 patients whose survival was greater than 1 month after SEMS placement. BMI in those patients was 19.7 ± 2.8 kg/m2 before SEMS placement and 20.2 ± 3.2 kg/m2 after SEMS placement and albumin level was 3.27 ± 0.60 g/dl before SEMS placement and 3.09 ± 0.59 g/dl after SEMS placement.
The mean follow-up period was 5.8 months (1-24 months).
Discussion and conclusionsIn our study, we reported technical success of 97.7% and clinical success of 88.3%, similar to that reported in other studies.12–27 Our results showed that 30.2% of the patients had SEMS dysfunction, a greater frequency than that reported in other studies. However, SEMS dysfunction in fewer than 6 months after placement occurred in only 7 (16.2%) of our patients, meaning it occurred before the expected half-life of the SEMS. That result corresponded to a similar frequency reported by Yamao et al.26 in a retrospective study of 278 patients with MGOO, in which SEMS dysfunction was 16.6%. In a study by Tringali et al.12 on 108 patients with MGOO, they reported SEMS permeability at 14 days in 94.6% of the patients, and utilizing the Kaplan-Meier estimator, SEMS permeability was 92.9% at 1 month, 86.2% at 2 months, 81.9% at 3 months, and 63.4% at 6 months, similar to the SEMS permeability at 6 months in 69.8% of our patients.
In our study, tumor ingrowth was the main cause of SEMS dysfunction (92.3%), similar to that reported in numerous studies.25 Endoscopic resolution of SEMS dysfunction in our patients was 63.8%, indicating that despite the high frequency of dysfunction, endoscopic re-intervention was generally feasible for and successful in re-establishing SEMS permeability.
The factors that predict SEMS dysfunction in MGOO are not well understood, and the results of published studies are contradictory. Yamao et al.,26 in their study on 278 patients with MGOO, reported that a KS < 50 was a predictor of SEMS dysfunction (p < 0.01). Sato et al.28 found that KS, peritoneal dissemination, and ascites were predictive factors of the clinical inefficacy of SEMS. Sasaki et al.29, in a retrospective study on 97 patients with MGOO, reported that a KS < 50 and ascites were predictive factors for the restriction of solids in oral food intake. Mendelsohn et al.14 concluded that ascites should not be a contraindication for duodenal SEMS, given that the clinical success of SEMS in patients with carcinomatosis was 81%. In our study, we found that diffuse gastric cancer and ucSEMS use were predictive factors for SEMS dysfunction in MGOO. Our results can be explained by the fact that, upon infiltrating the submucosa and the muscularis mucosae, diffuse gastric cancer conditions a decrease in gastric distensibility and affects gastric motility, favoring gastric retention. With respect to ucSEMSs as a dysfunction predictor, that could be related to the fact that tumor ingrowth was the main cause of SEMS dysfunction.
Numerous studies have compared covered SEMSs (cSEMSs) and ucSEMSs for the palliation of MGOO. Hori et al.27 conducted a retrospective study on 126 patients with ucSEMSs and 126 patients with cSEMSs and demonstrated that tumor ingrowth was more frequent in ucSEMS (ucSEMS 11.90% vs. cSEMSs 0.79%; p = 0.002) and SEMS migration was more frequent in cSEMSs (cSEMSs 8.73% vs. ucSEMSs 0.79%; p = 0.005). The KS (p = 0.04), absence of ascites (p = 0.02), and insufficient SEMS expansion (< 30%) (p = 0.003) were significantly associated with tumor ingrowth in ucSEMSs, whereas short SEMS length (p = 0.05) and chemotherapy (p = 0.03) were predictive of cSEMSs migration. Those authors concluded that ucSEMSs could be a good option in patients receiving chemotherapy and long cSEMSs a better option in patients in good general condition. In a randomized prospective study on 120 patients with MGOO, Lim et al.31 compared cSEMSs vs. ucSEMSs and found that the ucSEMS group had a greater frequency of tumor ingrowth (18.0% vs. 3.4%; p < 0.02), greater tumor overgrowth (3.4% vs. 3.3%; p < 0.99), and less SEMS migration (0% vs. 13.6%; p < 0.01). The authors of a randomized prospective study on 80 patients with MGOO showed a higher frequency of tumor ingrowth with ucSEMSs (25.0% vs. 0%; p < 0.01) and a lower frequency of migration at 8 weeks (25.8% vs. 2.8%; p < 0.01).32 In a study on 278 patients, Yamao et al.26 reported that ucSEMSs did not predict tumor ingrowth, but that cSEMS predicted migration (p < 0.01). In a meta-analysis that included 849 patients with MGOO, there were no significant differences in permeability and re-intervention rate between the cSEMS group and the ucSEMS group, but the cSEMSs were associated with a higher migration rate (RR: 3.48, 95% CI: 2.16-5.62, p < 0.00001) and a lower obstruction rate (RR: 0.42, 95% CI: 0.24-0.73, p = 0.002).33 The discrepancy between studies on that theme could be secondary to the different SEMSs utilized or to patient selection, which is why higher quality studies comparing cSEMSs and ucSEMSs in MGOO are required. In our study, we could not observe and evaluate SEMS migration as a cause of dysfunction because few pcSEMSs and no cSEMSs were utilized.
One of the main goals in patients with unresectable MGOO or those receiving palliative care is maintaining nutrition (ideally oral) because it is an important quality of life indicator. GJA provides an effective reduction of obstructive symptoms and enables oral intake resumption. Nevertheless, there are significant surgical risks for morbidity and mortality with surgical palliation. Studies have reported morbidity of up to 15.7% and mortality of up to 6.5%, being higher when combined with biliary bypass.34 SEMSs are less invasive, oral intake improves more quickly, and they are associated with fewer days of hospitalization.35,36 However, GJA can be a better option in patients with a life expectancy over 3 months, in whom numerous predictors of SEMS dysfunction have been identified.
With respect to nutrition, in our study, serum albumin was 3.23 ± 0.62 g/dl before SEMS placement and 3.05 ± 0.64 g/dl after SEMS placement, and BMI was 20.6 ± 3.2 kg/m2 before SEMS placement and 20.7 ± 3.3 kg/m2 after SEMS placement, suggesting that SEMSs have no impact on weight gain or serum albumin improvement. However, it appears that SEMSs enable initial weight and serum albumin level to be maintained, which is considered a relevant aspect in the quality of life of the patient receiving palliative oncologic care. We should keep in mind that the majority of those patients have ascites, which can cause a progressive increase in patient weight, making weight gain due to better oral intake indistinguishable from weight gain due to an increase in ascites.
The limitations of our study were its retrospective design, the fact that it was not randomized, and the small number of study patients, which reduced its statistical power.
In conclusion, SEMSs were shown to be effective palliative treatment for MGOO, with adequate technical and clinical efficacy. SEMS dysfunction was frequent and diffuse-type gastric cancer and ucSEMS use appeared to be dysfunction predictors. Said predictors can serve to carry out better patient selection for SEMS, but larger studies with better methodological quality are needed to establish the predictive factors of SEMS dysfunction in patients with MGOO.
Financial disclosureNo financial support was received in relation to the present study.
Conflict of interestThe authors declare that there is no conflict of interest.
Please cite this article as: Suder-Castro LS, Ramírez-Solís ME, Hernández-Guerrero AI, de la Mora-Levy JG, Alonso-Lárraga JO, Hernández-Lara AH. Predictores de disfunción del stent metálico autoexplandible en la obstrucción de salida gástrica maligna. Revista de Gastroenterología de México. 2020;85:275–281.