Some patients with autoimmune liver disease have characteristics of cholestasis, as well as of autoimmune hepatitis. Despite the fact that this is a relatively frequent clinical condition seen in referral centers for liver diseases, there is little evidence as regards the clinical management of these syndromes due to their low prevalence and the lack of standardized definitions and diagnostic criteria. This is relevant, given that published studies report that there is a lower therapeutic response and poorer outcome in patients with overlap syndrome than in those presenting solely with autoimmune hepatitis.
Whether overlap syndromes are distinct entities or the presence of 2 concurrent diseases is still a subject of debate. They should be suspected in autoimmune hepatitis patients that present with signs of cholestasis, as it is known that overlap behavior tends to be more aggressive, with higher rates of cirrhosis and the need for liver transplantation. Treatment response is also poorer and should be directed at the predominant component. Standardized definitions are necessary so that these syndromes can be studied in controlled clinical trials.
Algunos pacientes con enfermedad hepática autoinmune poseen características tanto de colestasis como de hepatitis autoinmune. A pesar de que es un escenario clínico relativamente frecuente en unidades de referencia para la atención de enfermedades hepáticas, debido a su baja prevalencia, la falta de estandarización en definiciones y en criterios diagnósticos, existe poca evidencia acerca de su manejo clínico, el cual es relevante ya que, de acuerdo a lo descrito, su respuesta terapéutica es menor y su pronóstico es peor que los de la hepatitis autoinmune aislada.
En la actualidad, aún existe controversia acerca de si los síndromes de sobreposición son entidades diferentes o la presencia de 2 enfermedades coexistentes. Deben ser buscados en los pacientes con hepatitis autoinmune que tienen datos de colestasis ya que sabemos que su comportamiento tiende a ser más agresivo con mayores tasas de cirrosis y necesidad de trasplante hepático así como pobre respuesta al tratamiento, el cual debe ser dirigido al fenotipo principal. Hacen falta definiciones estandarizadas que permitan su estudio en ensayos clínicos controlados.
Autoimmune liver diseases are a group of diseases characterized by an anomalous immune response directed at hepatocytes or bile ducts. Their importance lies in the fact that they can result in chronic liver damage with fibrosis and cirrhosis.1 They are classified in relation to their clinical, biochemical, serologic, histologic, and radiologic findings.
Autoimmune liver diseases are divided into 2 groups; the first is characterized by predominantly hepatocellular damage and its prototype is autoimmune hepatitis (AIH). The second is characterized by cholestasis and includes primary biliary cirrhosis (PBC) and primary sclerosing cholangitis (PSC).2–4
These diseases have been identified as clinical syndromes with their own diagnostic criteria and classic phenotypical presentations. However, there is no etiologic agent or characteristic pathogenic pathway whose measurement would enable us to make an accurate diagnosis.5 Evidence indicates that AIH, PBC, and PSC share pathophysiologic mechanisms. Even though these mechanisms are not yet fully understood, it is known that they are based on the interaction between genetic predisposition and triggering environmental factors that, associated with immunologic self-regulation defects, cause immunotolerance disruption and induce tissue damage mediated by antibodies and T-cells.6–8 In AIH, histologic damage is observed predominantly in the portal and periportal hepatocytes, whereas in PBC and PSC the histologic lesion affects the biliary epithelial cells, each with its distinctive characteristics.9
Overlap syndromes encompass a small group of patients within the spectrum of the autoimmune liver diseases that can have the characteristics of cholestasis (PBC or PSC) in combination with AIH.10 In overlap syndromes the characteristics of the coexisting diseases may present simultaneously or consecutively, resulting in the ongoing debate as to whether these syndromes should be considered distinct entities or variants of the primary diseases.11 Overlap syndromes apparently affect young AIH patients more frequently and their symptoms, which are very nonspecific, include fatigue, arthralgia, and pruritus.12
Due to the heterogeneous presentation of the overlap syndromes, the absence of standardized definitions and criteria, and the lack of controlled clinical trials, their treatment is largely empirical and extrapolated from the primary diseases.
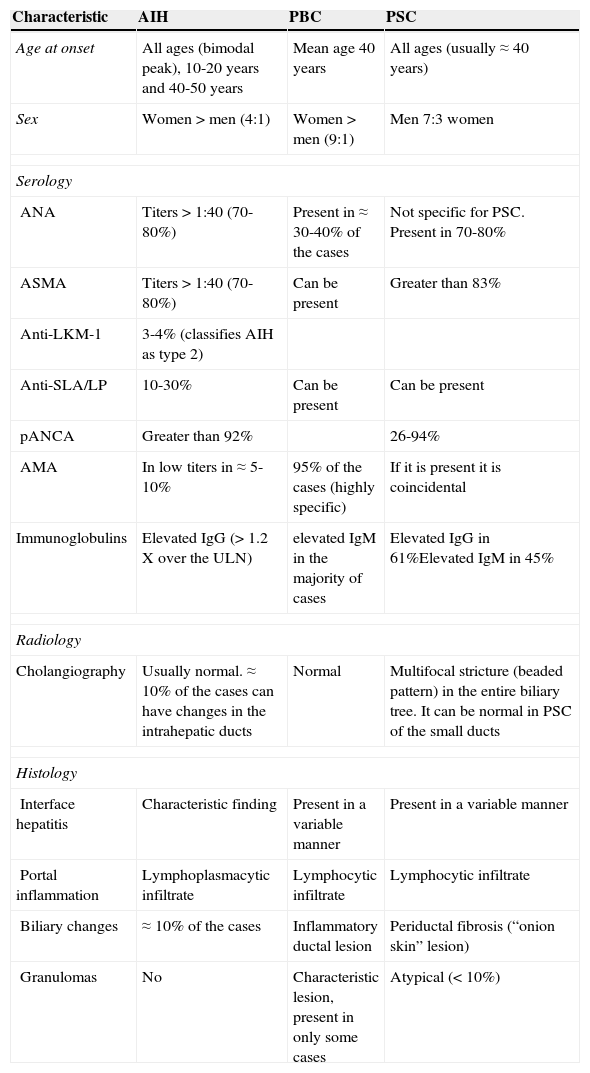
Types and diagnoses of overlap syndromesEach one of the autoimmune liver diseases has specific classic characteristics (table 1). Nevertheless, they are not completely homogeneous, and in relation to each disease, patients can present with a spectrum of different clinical, biochemical, serologic, and radiologic characteristics. Furthermore, the variability in the validation and reproducibility of the criteria and diagnostic tests must be taken into account, adding to the complexity of their classification and differential diagnoses.12
Characteristics of the patients with autoimmune liver diseases.
| Characteristic | AIH | PBC | PSC |
|---|---|---|---|
| Age at onset | All ages (bimodal peak), 10-20 years and 40-50 years | Mean age 40 years | All ages (usually ≈ 40 years) |
| Sex | Women > men (4:1) | Women > men (9:1) | Men 7:3 women |
| Serology | |||
| ANA | Titers > 1:40 (70-80%) | Present in ≈ 30-40% of the cases | Not specific for PSC. Present in 70-80% |
| ASMA | Titers > 1:40 (70-80%) | Can be present | Greater than 83% |
| Anti-LKM-1 | 3-4% (classifies AIH as type 2) | ||
| Anti-SLA/LP | 10-30% | Can be present | Can be present |
| pANCA | Greater than 92% | 26-94% | |
| AMA | In low titers in ≈ 5-10% | 95% of the cases (highly specific) | If it is present it is coincidental |
| Immunoglobulins | Elevated IgG (> 1.2 X over the ULN) | elevated IgM in the majority of cases | Elevated IgG in 61%Elevated IgM in 45% |
| Radiology | |||
| Cholangiography | Usually normal. ≈ 10% of the cases can have changes in the intrahepatic ducts | Normal | Multifocal stricture (beaded pattern) in the entire biliary tree. It can be normal in PSC of the small ducts |
| Histology | |||
| Interface hepatitis | Characteristic finding | Present in a variable manner | Present in a variable manner |
| Portal inflammation | Lymphoplasmacytic infiltrate | Lymphocytic infiltrate | Lymphocytic infiltrate |
| Biliary changes | ≈ 10% of the cases | Inflammatory ductal lesion | Periductal fibrosis (“onion skin” lesion) |
| Granulomas | No | Characteristic lesion, present in only some cases | Atypical (< 10%) |
AIH: autoimmune hepatitis; AMA: anti-mitochondrial antibodies; ANA: anti-nuclear antibodies; ASMA: anti-smooth muscle antibodies; LKM-1: live kidney microsomal antibodies; pANCA: perinuclear anti-neutrophil cytoplasmic antibodies; PBC: primary biliary cirrhosis; PSC: primary sclerosing cholangitis; SLA/LP: soluble liver antigen/liver pancreas; ULN: upper limit of normal.
Approximately 18% of the patients with autoimmune hepatopathy present with characteristics that are distinctive of a second autoimmune hepatic disease. The term overlap syndrome refers to the coexistence of 2 autoimmune diseases in the same patient.10,13 Thus, AIH can present with 3 cholestatic phenotypes that can be intermixed with its classic “hepatic” characteristics8 (table 2).
Diagnostic characteristics of the overlap syndromes.
| Overlap syndrome | Laboratory characteristics | Serologic characteristics | Histologic characteristics | Cholangiography |
|---|---|---|---|---|
| AIH/PBC | Paris criteria*Mild formsAP < 2 ULN | Positive AMA | Interface hepatitisDestructive cholangitis | Normal |
| AIH/PSC | AST/ALT > ULNγ-globulins and IgG > ULNAP or GGT > ULN | Negative AMA | Interface hepatitisDuctopeniaPortal edema or fibrosisFibro-obliterating cholangitis | Strictures/dilations(beaded pattern) |
| AIH/cholestatic syndrome | AST/ALT > ULNγ-globulins and IgG > ULNAP or GGT > ULN | Negative AMA | Interface hepatitisDestructive cholangitis or bile duct loss | Normal |
AIH: autoimmune hepatitis; ALT: alanine transaminase; AMA: anti-mitochondrial antibodies; ANA: anti-nuclear antibodies; AP: alkaline phosphatase; ASMA: anti-smooth muscle antibodies; AST: aspartate transaminase; GGT gamma glutamyltransferase; LKM-1: live kidney microsomal antibodies; pANCA: perinuclear anti-neutrophil cytoplasmic antibodies; PBC: primary biliary cirrhosis; PSC: primary sclerosing cholangitis; SLA/LP: soluble liver antigen/liver pancreas; ULN: upper limit of normal.
In relation to clinical practice, the presence of an overlap syndrome should be suspected when, in the setting of an autoimmune hepatopathy, there is a deviation in its natural progression, the biochemical and serologic patterns are not classic, or the therapeutic response is not the expected one.14
The nomenclature of the overlap syndromes has transformed over time. Specific terms used to be employed to define some of them (e.g. autoimmune sclerosing cholangitis for AIH/PSC overlap in children or autoimmune cholangitis for the AIH/undetermined cholestasis overlap). However, in order to avoid confusion and homogenize their classification, it is recommended to first name the dominant phenotype and then the secondary phenotype (e.g. AIH/PBC or AIH/PSC).
Unlike autoimmune liver diseases as isolated entities, overlap syndromes lack specific diagnostic criteria. Therefore, proposals to that end have been made that will be discussed further ahead. It is also recommended to define each of the primary autoimmune hepatopathies in accordance with the criteria that has been validated by the different international groups and associations, such as the International Autoimmune Hepatitis Group (IAIHG), the American Association for the Study of Liver Diseases (AASLD), and the European Association for the Study of the Liver (EASL).15
Autoimmune hepatitis overlap/primary biliary cirrhosisEpidemiology/diagnosisDue to its low frequency and the lack of standardized criteria, the prevalence of AIH/PBC overlap is difficult to establish. Nevertheless, it is estimated in 2-20% of the patients with AIH and 4.3-9.2% in those with PBC.16,17 It is important to mention that close to 10% of the patients with all the characteristics of AIH can persistently have positive anti-mitochondrial antibodies (AMAs), but the latter is not synonymous with PBC. O’Brien et al. studied 15 patients with AIH and positive AMAs whose histologic studies did not present with changes consistent with PBC.18 In another study on 130 patients with autoimmune hepatitis, 24 (18%) had positive AMAs. In that study, having positive AMAs was not associated with histologic biliary lesion, with ulterior PBC development, with different remission rates, or with treatment failure and therefore the presence of AMA was insufficient for diagnosing AIH/PBC overlap or predicting cholestatic phenotypes.19 Likewise, AMA positivity appears to be transitory, given that in the same study at patient follow-up 54% remained positive, 16% became negative, and the other 30% did not have positive AMAs at the onset of the study, but became positive during the follow-up period.19
AIH/PBC overlap diagnosis continues to be a challenge and there is no criterion standard. The most widely used criteria for its diagnosis come from 2 research groups: the so-called “Paris criteria” proposed by Chazouillères et al. in 199816 and the International Autoimmune Hepatitis Group (IAIHG).10 A recent study showed that the “Paris criteria” have a high sensitivity (92%) and specificity (97%) for AIH/PBC overlap diagnosis and thus are currently the most widely used.20
When utilizing the Paris criteria for establishing AIH/PBC overlap diagnosis, at least 2 of the 3 criteria per entity, with the following definitions, are required:11
For PBC:
- 1.
Alkaline phosphatase (AP) ≥ 2 times the upper limit of normal or gamma glutamyltransferase ≥ 5 times the upper limit of normal.
- 2.
AMA (≥ 1:40)
- 3.
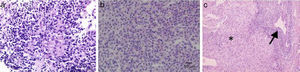
Liver biopsy with a florid bile duct lesion (degeneration of the bile duct epithelium with focal ductal obliteration and granuloma formation) (fig. 1).
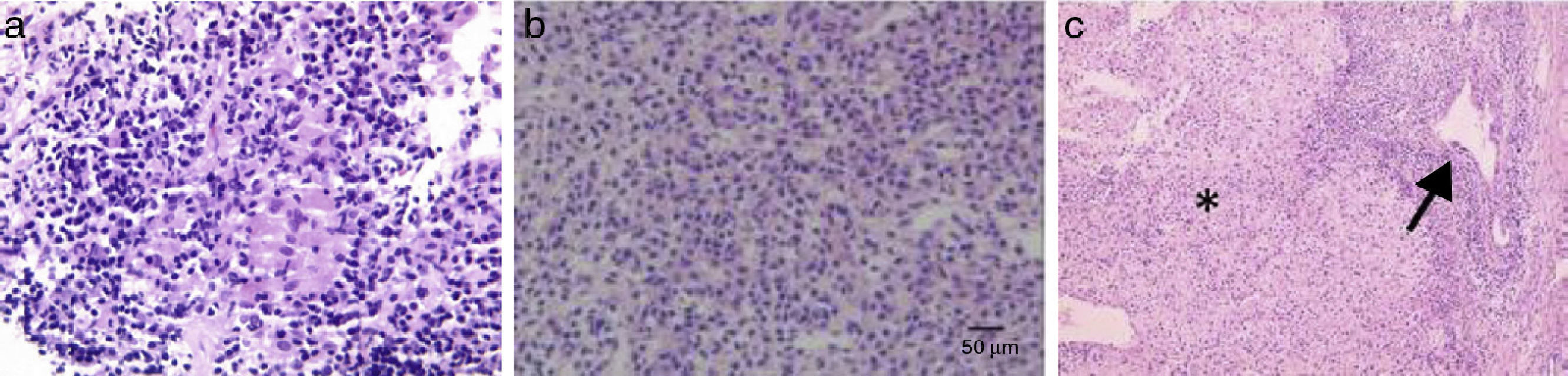
Figure 1.a. An epithelioid granuloma is observed in the portal tract, surrounded by lymphoplasmacytic infiltrate (granulomas are not typical of classic AIH). b. Destruction of interlobular bile ducts. c. Chronic non-suppurative destructive cholangitis (arrow) and interface and lobular infiltrate (asterisk).
(0.39MB).
For AIH:
- 1.
Alanine-amino-transferase (ALT) ≥ 5 times the upper limit of normal.
- 2.
IgG ≥ 2 times the upper limit of normal or positive anti-smooth muscle antibodies.
- 3.
Liver biopsy with periseptal or periportal lymphocytic necrosis.
On the other hand, the IAIHG suggests applying the diagnostic score for AIH to patients with PBC to identify the cases with AIH characteristics. Since 1993 the IAIHG diagnostic criteria have been revised and in 2008 they were simplified, establishing a score ≥ 7 points as definitive AIH and ≥ 6 as probable AIH. By not giving the AMAs or the bile duct histologic lesion a negative score, the simplified score appears to better identify the patients with overlap than its extensive counterpart.21
In relation to the serologic profile of AIH/PBC overlap syndrome, Muratori et al. compared 240 patients (120 with pure PBC and 120 with pure AIH) with 15 patients that had AIH/PBC overlap according to the Paris criteria and found that the concomitant presence of AMA and anti-nuclear antibodies was a highly specific pattern for AIH/PBC overlap.22
On the other hand, despite the fact that the etiology of autoimmune liver diseases is not yet understood, it is known that various environmental factors act in combination with the genetic susceptibility of the individual to alter the immune response. With respect to this, the presence of AIH has been associated with the major histocompatibility complex, particularly with the HLA-DR3/DR4 and HLA-B8 loci,23 but the data on AIH/PBC overlap are limited. In 2011 a study on this group of patients found differences between the HLA-DR alleles; the presence of HLA-DR7 was more frequent in patients with overlap than in those with AIH, which could help distinguish those patients with AIH that are prone to present with AIH/PBC overlap syndrome.24 Another study conducted on Mexican patients found that the patients with PBC had a higher frequency of HLA-DR4 and DR1 alleles, when compared with healthy controls and patients with overlap syndromes. On the other hand, the healthy controls had a higher frequency of the HLA-DR5 allele, suggesting that the latter could play a protective role in the development of liver autoimmunity.25 There is little information in regard to this and therefore further studies are needed to confirm these findings.
Treatment/outcomeIt is a well-established fact that for patients with PBC, ursodeoxycholic acid (UDCA) at a dose of 13-15mg/Kg/d slows the progression of fibrosis and the instauration of end-stage liver disease, mainly in those patients with biochemical improvement. On the other hand, immunosuppressants are the treatment cornerstone in AIH, and its aim is to achieve complete disease remission for the purpose of preventing and/or reverting liver fibrosis progression.26,27
Unfortunately, the lack of validated criteria and the scarcity of clinical trials means that AIH/PBC treatment has to be empirical, based on data extrapolated from primary autoimmune liver diseases and sustained in retrospective trials and case series.5,28 With said limitations, the EASL and IAIHG guidelines recommend the combination of immunosuppressant drugs (corticosteroids/azathioprine) with UDCA at a dose of 13-15mg/kg/d as a first therapeutic option. As an alternative form in those patients with mild AIH activity, UDCA can be used as monotherapy, adding immunosuppressive therapy at 3 months if there has been no biochemical response.
Information in relation to outcome in patients with AIH/PBC overlap is variable. Some studies have documented a worse outcome with a greater progression to cirrhosis and complications due to portal hypertension, when compared with PBC as a single entity. 29–30 In contrast, other reports describe a better outcome with respect to progression to fibrosis, overall survival, and transplant-free survival mainly when the combination treatment is used and there is good biochemical response.5,31
In conclusion, AIH/PBC diagnosis continues to be a challenge, the Paris criteria are currently a good diagnostic tool, and while there are no large-scale, randomized, and prospective clinical trials, the combination treatment (immunosuppressants and UDCA) is recommended. Those patients that do not fit the Paris criteria for overlap should receive treatment in accordance with the predominant phenotype.
Autoimmune hepatitis/primary sclerosing cholangitis overlapDefinitionAIH/PSC overlap is another of the cholestatic phenotypes of AIH and is characterized by having negative AMAs and cholangiographic alterations in NMR cholangiography or endoscopic retrograde cholangiography.8 In patients with AIH/PSC overlap there is an entity known as autoimmune sclerosing cholangitis that presents in children and is defined the same as AIH/PSC in adults, except that compared with the latter, its treatment response is better. In fact, approximately 50% of the children with AIH have cholangiographic alterations.32
Patients with PSC can have altered aminotransferases, hyperglobulinemia, and positive antibodies, as well as interface hepatitis in biopsy in up to 30% of patients.10
A syndrome worth mentioning only due to its rareness is PBC/PSC. Its frequency has been estimated at 0.7% in a cohort of 261 patients with autoimmune liver disease that has been followed for 20 years.15,33
EpidemiologyThe existing variability in frequencies is most likely a reflection of the characteristics of the cohorts and the diagnostic method employed.34
The approximate frequency of PSC criteria in patients with AIH ranges from 6-11%8 and the prevalence of autoimmune hepatitis in patients with PSC as the dominant phenotype is from 2-33%.13 Upon applying the revised IAIHG criteria to patients with PSC, the prevalence of AIH criteria is from 7-14%.
On the other hand, the prevalence of cholangiographic alterations in patients with AIH is variable, depending on the population studied, and is 2-10% in adults with classic AIH, 41% in adults with AIH and UC, and 50% in children with AIH.15
A study on 79 patients with AIH found cholangiographic alterations through NMR cholangiography that were consistent with PSC in 10% of the patients. The patients with cholangiographic alterations had a lower age at the time of diagnosis and higher bilirubin and alkaline phosphatase figures than the patients with no cholangiographic alterations.35
Not all the cholangiographic alterations in NMR cholangiography indicate the presence of AIH/PSC overlap. This was illustrated in a study that compared 24 patients presenting with AIH and cholangiographic alterations through NMR cholangiography with 27 controls presenting with non-autoimmune liver cirrhosis. That study found that the only predictor of cholangiographic alterations was the presence of liver fibrosis, given that the proportion of patients with cholangiographic alterations in the intrahepatic bile ducts was had greater similarity to patients with AIH and significant fibrosis (46%) than to the controls with non-autoimmune cirrhosis (59%). These findings suggest that in a large number of patients with AIH, the cholangiographic alterations seem to be more related to the presence of fibrosis than to an actual AIH/PSC diagnosis.36
It has consistently been stated that AIH and PBC are more frequent in women and that PSC is more frequent in men. Interestingly, that tendency is maintained in AIH/PSC overlap, with approximately 62% of the cases presenting in men.13
PathogenesisAIH and PSC share a genetic background. A high prevalence of HLA-B8 and DR3 has been observed in both diseases, whereas the presence of HLA-DR4 appears to predispose to AIH and protect from PSC. The HLA-DR52 allele seems to be associated with PSC: In relation to AIH/PSC overlap syndrome, the published literature indicates that DR3 frequency is similar to that of patients that have only PSC or AIH.13
The evidence suggests that the diseases frequently develop sequentially. This was illustrated in a study on 238 patients with AIH in which 16 (6%) met the AIH/PSC overlap criteria.37 Said study reported that in approximately 50% of the patients with AIH/PSC, the initial diagnosis was AIH with a later development of PSC cholangiographic changes and in the other 50% the 2 diseases presented initially.37 In another study on 16 patients with AIH/PSC, 62% had initially been diagnosed with AIH and later developed PSC characteristics, 18% of patients with PSC later developed AIH characteristics, and 18% had the criteria of both diseases at the onset. It is striking that in this last study the cases were reviewed retrospectively and they found biochemical and histologic characteristics of both diseases in 100% of the patients at the onset, regardless of the initial diagnosis; this suggests that the biochemical phenotype is initially different in the diseases as single entities.38 On the other hand, it is uncommon that patients with PSC later develop AIH characteristics.10,15
DiagnosisAIH scores are not useful for defining its cholestatic phenotypes and liver biopsy together with clinical judgment are the best tools at present.34
In relation to the biochemical parameters, classic AIH tends to have higher transaminase values compared with AIH/PBC and AIH/PSC, and AIH/PSC tends to have higher AP values than classic AIH and AIH/PBC, although there is important overlap of said values and therefore these parameters do not appear useful for their differenctiation.37
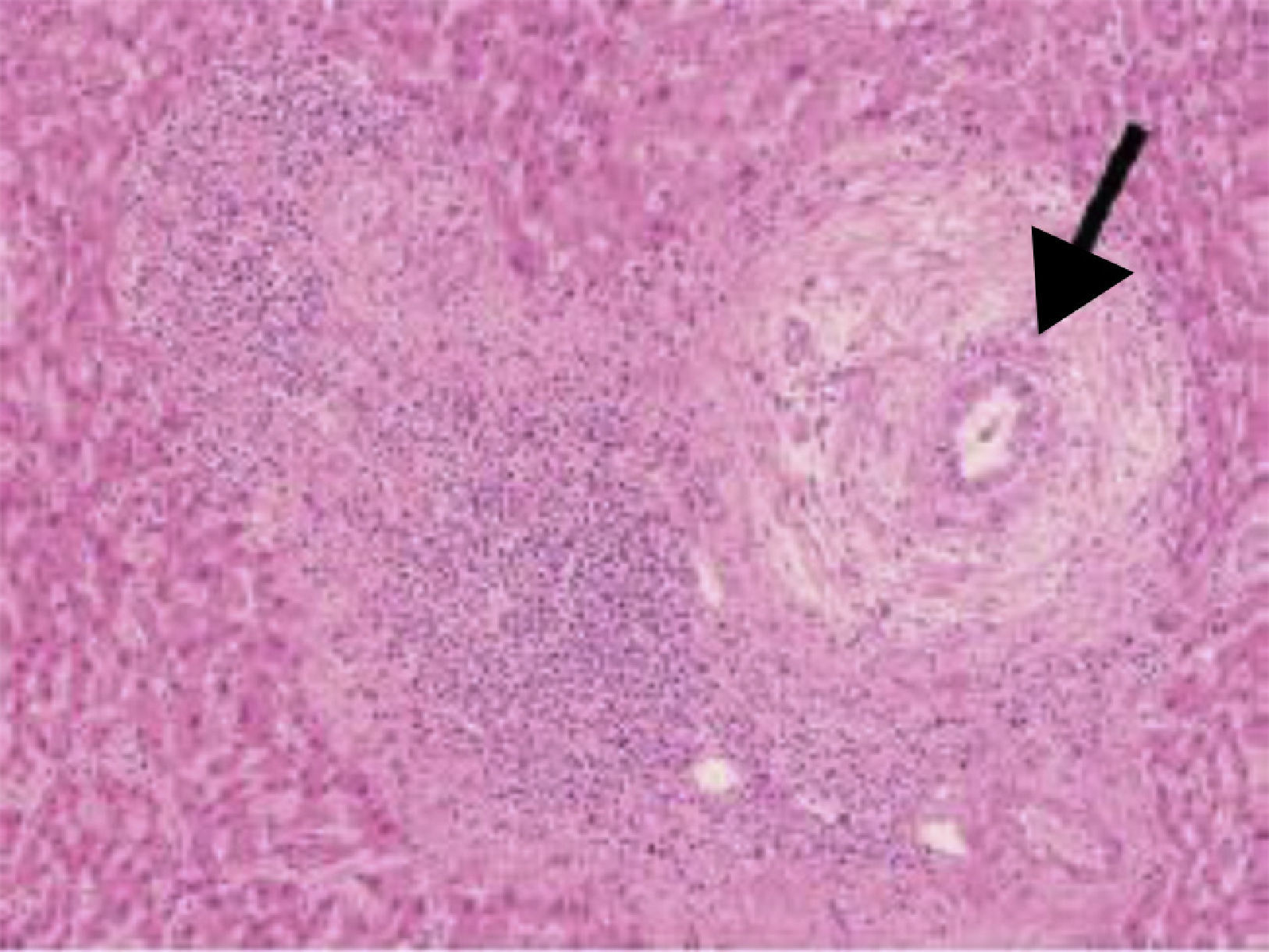
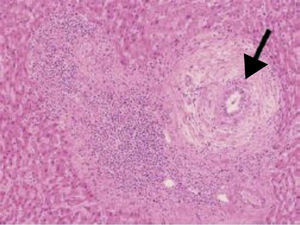
In histology, interface hepatitis, portal edema or fibrosis, ductopenia, ductular proliferation, or fibro-obliterating cholangitis can be observed8 (fig. 2).
The antibody profile is variable. In a study on 16 patients with AIH/PSC overlap, 81% of the patients were found to be positive for pANCA, 62.5% for antinuclear antibodies, and 50% for SMA.38
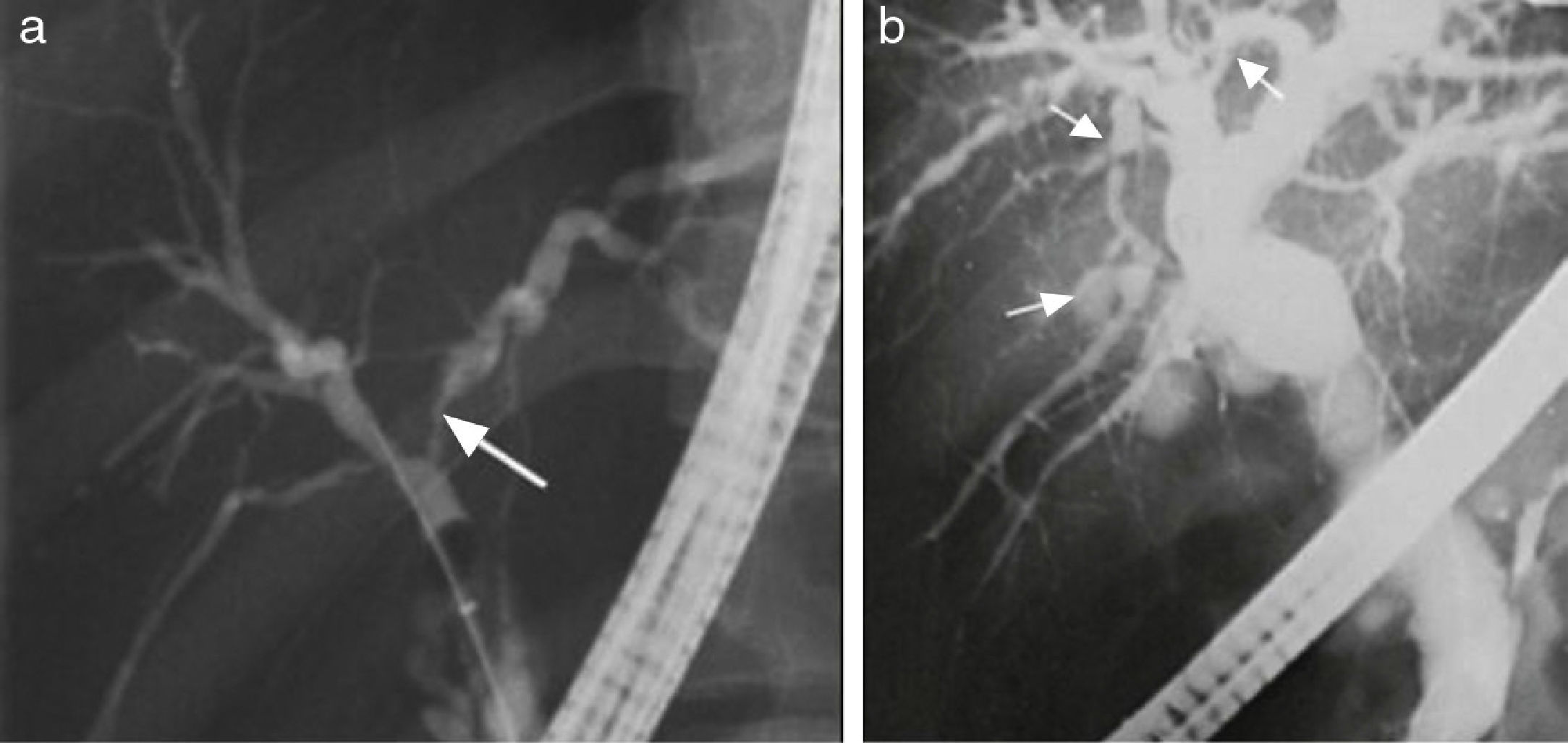
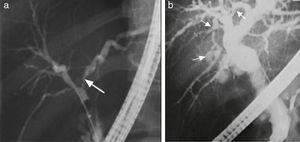
In patients meeting the AIH diagnostic criteria, imaging studies that show regeneration nodules > 3cm, peripheral atrophy of the hepatic parenchyma, and a beaded cholangiographic pattern (fig. 3) have a specificity of 100% for AIH/PSC overlap syndrome.39 Those findings appear to be attributed to the PSC component because they have also been observed in PSC as a single entity. It is worth mentioning that the presence of cirrhosis can alter the architecture of the hepatic ducts and consequently the imaging patterns.39
Cholangiographic changes appear to be expressed as late changes. This was shown in a study on 16 patients with AIH/PSC in which, despite having histologic PSC and biochemical cholestatic changes, none of the patients that underwent imaging studies had cholangiographic changes; they developed during follow-up in 87% of the patients.38 In another prospective 5-year study, 41 patients with PSC were followed, 7 of whom had AIH/PSC overlap (17%). In all the patients, initial diagnosis was immunosuppressant-resistant AIH for which endoscopic cholangiography was carried out. It revealed cholangiographic changes at a median of 17 months after AIH diagnosis.40
Inflammatory bowel disease is frequent in patients with AIH/PSC (approximately 25%).38 It is known that 41% of the patients that present with AIH and UC have cholangiographic lesions. This justifies the performance of cholangiography in this patient subgroup, unlike the patients with AIH that do not have inflammatory bowel disease, whose prevalence of cholangiographic lesions is 10%.8
AIH/PSC overlap should be suspected in adults diagnosed with AIH that have poor therapeutic response to steroids, cholestasis, histologic biliary lesion, absence of AMAs, and coexisting inflammatory bowel disease. It is thus reasonable to order an NMR cholangiography if any of those characteristics are identified.
TreatmentThere is no uniform treatment and it should be individualized.32 There are also non-controlled clinical trials in patients with AIH/PSC overlap and the medical treatments analyzed in classic PSC have not had good results, given that none have demonstrated benefit in crude or transplantation-free survival.15
The EASL consensus10 and the AASLD guidelines4 for PSC recommend that patients with AIH/PSC overlap be treated, along with endoscopic therapy when indicated, with the combination of azathioprine and prednisone, at doses of 1-2mg/kg and 0.5mg/kg, respectively. The prednisone dose should be gradually reduced until reaching 10-15mg/d. UDCA 13-15mg/kg/d is also recommended, underlining the fact that responses are inconsistent and evidence is not solid. In a clinical trial conducted on 150 patients with PSC, 2 therapeutic arms were randomly carried out; the first received UDCA at a dose of 28-30mg/kg and the second received placebo. The study was suspended after 6 years due to therapeutic futility and the outcomes were evaluated. Despite having higher improvement rates in AST and alkaline phosphatase levels, the patients in the UDCA group showed a greater risk for cirrhosis, varices, liver transplantation, or death, as well as higher severe adverse effect rates than the patients in the placebo group. Thus, despite their not having been studied in relation to AIH/PSC overlap,41 high doses of UDCA (28-30mg/kg) are not recommended.8,10,15
Steroid treatment is usually ineffective in adult patients with AIH/PSC of the large and small ducts, in patients with and without inflammatory bowel disease. Compared with patients that present with classic AIH, those with AIH/PSC overlap have a lower biochemical and histologic remission rate (22 vs 64%), a higher rate of deterioration during treatment (33 vs 10%), and a higher rate of death or need for liver transplantation (33 vs 8%). In addition, treatment response appears to be slow; in a study on 16 patients with AIH/PCS38, ALT level normalization took approximately 26 months.
The only subgroup of patients with AIH/PSC with acceptable rates of response to steroids (approximately 89%) and low rates of progression to cirrhosis are children. In fact, their results could be considered similar to the response seen in pure AIH.10,32
Finally, a little-used and little-studied therapy in AIH overlap syndromes is mycophenolate mofetil.42 In a Dutch study on 45 AIH patients, 15 of them had AIH/PSC overlap and 11 had AIH/PBC overlap. The remission rate was 57% in patients that did not respond to azathioprine and 63% in patients with azathioprine-intolerance.42
PrognosisIn general, therapeutic response and outcome is better in patients with AIH/PBC than with AIH/PSC.32
In a study on 238 patients with AIH in which 16 (6%) had AIH/PSC overlap, survival in the AIH/PSC patients was significantly lower than in the patients with AIH or AIH/PBC overlap.37 On the other hand, the patients with AIH/PSC appeared to have a better prognosis than the patients presenting with PSC alone.10 The latter is exemplified in a study in which the patients with AIH/PSC treated with immunosuppression apparently had better survival than the patients with classic PSC treated with UDCA.10,37,43
The clinical behavior of AIH/PSC appears to be different from that of AIH or PSC as separate entities because, on the one hand, treatment response is lower and fibrosis development is higher compared with classic AIH, and on the other hand, inflammatory bowel disease prevalence and the development of cholangiocarcinoma appear to be lower than in PSC alone. In a prospective study on 7 patients with AIH/PSC, none of the patients developed cholangiocarcinoma in an 88-month follow-up period.15,38,40 In said study, the patients with classic PSC showed greater disease progression, a higher liver transplantation rate, a higher rate of intrahepatic and extrahepatic neoplastic development, and a higher mortality rate compared with the patients with AIH/PSC overlap.40
Reduced aminotransferase levels do not appear to adequately predict outcome, given that in a study on 16 patients with AIH/PSC, despite achieving normalization of ALT levels in 87% of the patients, 56% had cirrhosis in follow-up liver biopsies and at the end of the study; after a 12-year follow-up period, 75% of the patients presented with cirrhosis.15,32,38 On the other hand, in relation to PSC, AP was shown to have a prognostic value, given that normal values or their reduction to < 40% of their initial value, was associated with better survival.34
Early-stage disease and the absence of cholangiographic alterations in the large ducts alone, compared with the small ducts alone, appear to be favorable outcome factors.32
Autoimmune hepatitis overlap/undetermined cholestasisDefinitionAIH overlap/cholestatic syndrome is also known as autoimmune cholangitis and consists of histologic lesion or loss of the bile ducts, negative AMAs, and normal cholangiography. This syndrome can include seronegative PBC and small duct PSC.8,15
EpidemiologyThe approximated frequency of an undetermined cholestatic phenotype in patients with AIH ranges from 5-11%.8
DiagnosisThe diagnosis of AIH/undetermined cholestasis is made when there is AIH with data of cholestasis and histologic bile duct damage, negative AMAs, and normal cholangiography.15
Histologic changes in AIH/cholestatic syndrome can be portal fibrosis, portal edema, and ductopenia characteristic of PCS, or lymphoplasmacytic infiltrate with interface hepatitis and bile duct lesion suggestive of PBC.8,15,44
In patients with AIH/undetermined cholestasis, upon carrying out ample serologic evaluations that utilize enzymatic immunoassays based on the gp210 and sp100 antigens and mitochondrial components, positivity for said antibodies has been found in 35% of the patients with negative AMAs, which would support seronegative PBC diagnosis.34
Treatment and prognosisTreatment with immunosuppressant drugs or UDCA usually is not effective in patients with AIH/undetermined cholestasis overlap, with a therapeutic failure of 88-100% and need for transplantation in 33% of the patients.15,17,44,45 This poor response is worse in classic AIH and AIH/PSC overlap of the large ducts.
Differential diagnosesInfiltration by IgG4 + plasma cells (> 5 per high power field) has been described in 3-35% of the patients with AIH and this could be part of a spectrum that includes AIH, autoimmune pancreatitis, and sclerosing cholangitis. The usual phenotype in this group of patients is IgG4-related autoimmune hepatitis as the dominant component and PSC as the clinical subcomponent.32 IgG4-related cholangitis is a systemic disease causing hepatitis as well as sclerosing cholangitis and can be confused with an overlap syndrome. It is characterized by elevated IgG4 and positive IgG4 plasma cell infiltration into the bile ducts and hepatic parenchyma. In addition, it can be associated with autoimmune pancreatitis and IgG4-related systemic disease that involves the salivary glands, retroperitoneum, gastrointestinal tract, lymph nodes, kidneys, and lungs.15
IgG4-related cholangitis should be suspected in male patients, older adults without inflammatory bowel disease, with strictures that are predominantly in the distal choledochus, imaging abnormalities in the pancreas, or multiorgan involvement with data of hepatitis/sclerosing cholangitis. Approximately 9-22% of the patients with classic PSC have modest increases in IgG4 (> 140mg/dL), but an IgG4 value > 2 ULN is highly specific for IgG4-related cholangitis. It is important to mention that not all patients with IgG4-related cholangitis have elevated serum levels of that biomarker, and so it should also be looked for in the tissue for the histopathologic analysis.11,15,46–50.
Various drugs and herbal remedies can also cause biochemical and histologic findings of hepatitis/cholangitis, as well as the presence of antibodies.10,15
The drugs associated with chronic hepatitis with the presence of antibodies are minocycline, nitrofurantoin, hydralazine, methyldopa, halothane, diclofenac, isoniazid, and infliximab. On the other hand, the drugs associated with mixed data of cholestasis and hepatocellular damage are various antibiotics (amoxicillin/clavulanate, trimethoprim/sulfamethoxazole, erythromycin, fluoroquinolones, and tetracyclines), antifungals (ketoconazole and itraconazole), antivirals (stavudine, didanosine, and nevirapine), NSAIDs (piroxicam, diclofenac), immunomodulators (azathioprine), psychotropics (chlorpromazine, risperidone, imipramine, amitriptyline), and others, such as captopril, glibenclamide, phenytoin.15
Herbs and dietary supplements that have been associated with hepatitis alone or cholestatic hepatitis are various Chinese herbs (Jin Bu Huan, Dai-saiko-to), black cohosh, green tea, euphorbia, chaparral, and some Herbalife products.15
Despite the suspension of said drugs, some patients may progress to the infrequent entity of chronic intrahepatic cholestasis that simulates PBC, also known as evanescent bile duct syndrome that is characterized by chronic bile duct lesion, ductopenia, and progression to cirrhosis.15,51
Finally, there are other disorders among the differential diagnosis of biochemical alterations consistent with hepatitis and/or cholestasis, such as the cholestatic varieties of viral hepatitis, steatohepatitis (alcoholic and non-alcoholic), infiltrating disorders, paraneoplastic syndromes, any cause of liver cirrhosis, idiopathic ductopenia in the adult, and any cause of secondary sclerosing cholangitis.11
Liver transplantation and overlap syndromesThere are few studies documenting the outcome of patients with overlap syndromes that have undergone transplantation.8,15,52
Approximately 25% of liver transplantations are performed in patients with autoimmune liver diseases and the prevalence of disease recurrence increases over time. Five-year recurrence is 17-42% for AIH, 12-13% for PBC, and 12-60% for PSC.52
In a study with 231 patients that underwent liver transplantation due to autoimmune liver disease, 5% had overlap syndrome (58% AIH/PBC and 42% AIH/PSC).52 In these patients, having overlap syndrome was found to be an independent risk factor for recurrence in the graft. Approximately 69% of the patients with overlap syndromes had recurrence in the graft, compared with approximately 30% recurrence in patients with an autoimmune liver disease alone. In addition, recurrence time was shorter (67 months in overlap syndromes vs > 135 months in the patients with a single disease). It was striking that in 70% of the patients with a pre-transplantation diagnosis of overlap syndrome, recurrence developed as a single disease in 70% and as overlap in only 30%. It is not known whether these patients will develop overlap characteristics as time goes by. The higher relapse rates in the graft in patients with overlap syndromes do not appear to result in greater graft loss or lower survival.52 Furthermore, a Japanese study reported that survival rates for AIH and PBC/AIH were excellent and there was no evidence of recurrence in the following 6-year follow-up period.15,53
ConclusionsOverlap syndromes in the autoimmune liver diseases are few and there is a lack of standardized definitions. At present it is not known if they are separate entities or if they form part of the classic diseases. However, it is important to recognize them because their clinical course is more aggressive and they have a lower therapeutic response. Therapeutic evidence is scarce in regard to overlap syndromes and the recommended treatments are based on retrospective studies and extrapolated from the experience in patients with classic diseases. Randomized clinical trials are lacking and it is hoped that better quality evidence on this theme will be produced in the near future.
Ethical responsibilitiesProtection of persons and animalsThe authors declare that no experiments were performed on humans or animals for this study.
Data confidentialityThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Financial disclosureNo financial support was received in relation to this study/article.
Conflict of interestThe authors declare that there is no conflict of interest.
The authors wish to thank Francisco Daniel Briseño García, Gastroenterology Resident at the Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán, SSA, Mexico City, Mexico.
Please cite this article as: Aguilar-Nájera O, Velasco-Zamora JA, Torre A. Diagnóstico y tratamiento de los síndromes de sobreposición de hepatitis autoinmune. Revista de Gastroenterología de México. 2015;80:150–159.