El objetivo del Consenso Mexicano de Hipertensión Portal fue desarrollar un documento guía para facilitar la práctica clínica en eventos clave del paciente con hipertensión portal y sangrado variceal. El panel de expertos incluyó gastroenterólogos, hepatólogos y endoscopistas mexicanos distinguidos por su trayectoria profesional. El documento exploró temas de interés en los siguientes módulos: profilaxis preprimaria y primaria, hemorragia variceal aguda y profilaxis secundaria. El manejo del sangrado variceal ha mejorado notablemente en años recientes. La información actual indica que el manejo general del paciente cirrótico con sangrado variceal se debe realizar por un equipo multidisciplinario, lo que tiene un papel importante en el desenlace final. Se recomienda combinar la terapia farmacológica y endoscópica en el manejo inicial; los fármacos vasoactivos se deben iniciar cuanto antes ante la sospecha de sangrado de origen variceal y mantenerse durante 5 días. Después de estabilizar al paciente, se realizará la endoscopia diagnóstica de urgencia por un endoscopista calificado, y se dará el tratamiento endoscópico variceal correspondiente. La profilaxis con antibiótico se debe considerar como parte integral del tratamiento, iniciarse desde el ingreso hospitalario y mantenerse durante 5 días. En caso de falla terapéutica, las terapias de rescate se deben iniciar de inmediato; tomando en cuenta que las terapias de derivación mediante radiolgía de intervención son muy efectivas en el control del sangrado variceal refractario. Estas guías están basadas en la mejor evidencia disponible sobre hipertensión portal, y están dirigidas a lograr una mayor eficacia clínica.
The aim of the Mexican Consensus on Portal Hypertension was to develop documented guidelines to facilitate clinical practice when dealing with key events of the patient presenting with portal hypertension and variceal bleeding. The panel of experts was made up of Mexican gastroenterologists, hepatologists, and endoscopists, all distinguished professionals. The document analyzes themes of interest in the following modules: preprimary and primary prophylaxis, acute variceal hemorrhage, and secondary prophylaxis. The management of variceal bleeding has improved considerably in recent years. Current information indicates that the general management of the cirrhotic patient presenting with variceal bleeding should be carried out by a multidisciplinary team, with such an approach playing a major role in the final outcome. The combination of drug and endoscopic therapies is recommended for initial management; vasoactive drugs should be started as soon as variceal bleeding is suspected and maintained for 5 days. After the patient is stabilized, urgent diagnostic endoscopy should be carried out by a qualified endoscopist, who then performs the corresponding endoscopic variceal treatment. Antibiotic prophylaxis should be regarded as an integral part of treatment, started upon hospital admittance and continued for 5 days. If there is treatment failure, rescue therapies should be carried out immediately, taking into account that interventional radiology therapies are very effective in controlling refractory variceal bleeding. These guidelines have been developed for the purpose of achieving greater clinical efficacy and are based on the best evidence of portal hypertension that is presently available.
Introduction
Portal hypertension is currently defined as the increase in the portosystemic pressure gradient in any segment of the portal venous system. Even though portal hypertension can result from prehepatic alterations (portal vein or splenic vein hypertension), posthepatic alterations (Budd-Chiari Syndrome), or non-cirrhotic intrahepatic causes (schistosomiasis, sinusoidal obstruction syndrome), hepatic cirrhosis is the most common cause of portal hypertension and thus is the most widely studied. A hepatic venous pressure gradient (HVPG) of 10 mmHg or higher has been documented to identify a group of patients with a more aggressive clinical course, such as the development of gastric and esophageal varices, clinical decompensation (the development of ascites, variceal hemorrhage, and encephalopathy), complications after hepatic resection (decompensation or death thereafter), and the development of hepatocellular carcinoma.
Variceal bleeding in the cirrhotic patient is the most direct complication of portal hypertension and results in a high morbidity and mortality rate. However, recent data suggest an improvement in these indicators through the advances in prophylaxis and treatment, when compared with previously reported studies.1-5 Nevertheless, some studies6 show that clinical physicians to not closely follow the management guidelines for the patient with acute variceal bleeding. There are no data corresponding to the Mexican health system, but similar or worse figures could be expected, given the lack of available guidelines in Spanish.
The information in this area has been managed largely through consensus conferences among experts in which the events and outcomes have been defined and the existing evidence has been carefully reviewed, resulting in practical recommendations. The first of these conferences took place in 1986 in Groningen, Holland, and since then these reunions have alternated between Europe (the Baveno conference) and the United States (the American Association for the Study of Liver Diseases).
This situation illustrates the growing need for a Mexican Consensus with guidelines based on the best current evidence for the management of patients presenting with portal hypertension. This consensus in Spanish aims to improve the clinical practice regarding portal hypertension in Mexico.
Methodology
In the first stage, a working committee was formed that proposed the consensus methodology and elaborated a basic questionnaire. This preliminary document was then transformed into the final version of the "Portal Hypertension Treatment Guidelines".
This committee included Mexican specialists in gastroenterology, endoscopy, and hepatology.
The basic questionnaire formulated by the working committee was made up of statements that examined the following 3 modules of knowledge: preprimary and primary prophylaxis, acute hemorrhage, and secondary prophylaxis.
These statements were reviewed, discussed, and finally approved during the work meetings, and in this manner the basis for writing the future treatment guidelines was laid out.
Delphi panel-type consensus dynamic
The answer to each statement was manifested according to the Delphi Panel-type dynamic that scores the agreement/ disagreement on a scale from 1 to 9 with the option to add proposed changes to the statements.
The answers with a score of 6 or higher were regarded as being "in agreement" with the statement. Each of the answers was based on evidence found in national and/or international publications.
The organizing Committee received the panelists' responses to the specific statements and those in which there was no consensus (less than 60% agreement) were re-evaluated in order to write a new statement based on scientific evidence, which was proposed at the "Consensus Face-to-Face Meeting".
Finally, a working draft called the "Mexican Consensus Proposals" was prepared and given to each panelist at the Meeting/Workshop that was held in April 2010 in Hermosillo, Sonora, Mexico. The contributions that came out of the Baveno V Consensus, published in October 2010,7 were incorporated into the final document by a special work group and approved by all the authors.
At this meeting, the coordinators presented the statements to be evaluated for the final consensus. Those in which there was no consensus were defined as "no agreement" and put on hold until the availability of further scientific evidence.
All the participants were asked to put their levels of agreement in writing, to be published as the final guidelines in the REVISTA DE GASTROENTEROLOGÍA DE MÉXICO.
First module. Preprimary and primary prophylaxis in portal hypertension
Introduction
Portal hypertension, pressure above 5 mmHg, causes the development of esophageal varices (EV). These are regarded as one of the most important complications; they form portosystemic collaterals that are responsible for variceal bleeding.8,9 Therefore, it is a variable that defines the progression of cirrhosis from compensated to decompensated. Even with current treatments, the morbidity and mortality associated with this condition is high, emphasizing the need for more effective preventive treatment.3
At the time of cirrhosis diagnosis, varices are present in 30-40% of compensated patients and in 60% of decompensated patients.10-12 In those cirrhotic patients that do not present with varices in their first endoscopy, the annual incidence of EV is from 5 to 10%.13-15
Pathophysiology
The formation of varices is a direct consequence of the increase in portal pressure that, in cirrhosis, is caused by an increase in both the resistance to portal flow and portal venous affluence. The increase in resistance is as much structural (distortion of the hepatic vascular architecture from fibrosis and regenerative nodules) as it is dynamic (with myofibroblast contraction, stellate cell activation causing an increase in vascular tone due to endothelial dysfunction and a decrease in the bioavailability of nitric oxide, and an elevated activity in endogenous vasoconstrictors such as endothelin, alpha-adrenergic stimulus, and angiotensin, among others).16,17 This increase in pressure is the initial factor that leads to the opening of pre-existent embryonic vascular channels. In addition, there appears to be an elevated expression of angiogenic factors in the splanchnic vasculature, as the expression of VEGF.18-20
When there is a significant rise in the HVPG, collaterals are developed at communication sites between the portal and systemic circulations.10 This process is modulated, as mentioned before, by angiogenic factors.21,22 Concomitantly, the increased portal venous flow, as a result of splanchnic vasodilatation and increased cardiac output,23 maintains and raises portal hypertension.
Risk for bleeding is closely correlated with the degree of portal pressure. EV are the most frequent and clinically relevant, and are formed when the HVPG exceeds 10 mmHg.24 A reduction of at least 20% of the HVPG baseline value or a HVPG less than 12 mmHg, significantly reduces the risk for bleeding.25 Inter ventions that help reduce this pressure have been shown to prevent bleeding.
Clinical course and outcome
Variceal bleeding is the last step in a series of events that begin with an increase in portal pressure, followed by the development and progressive dilatation of varices until they finally burst and bleed. The appearance of varices in compensated patients indicates a change in clinical stage, from the very low stage of death at one year (1%), to a stage of intermediate risk (3.4%). The appearance of variceal bleeding is a catastrophic event with a very high risk for death at one year (57%).26
The endoscopic classification of EV is somewhat subjective and is susceptible to interobser ver variability.27-29 Of the existing classification systems, the one developed by the North Italian Endoscopy Club in 1988 divided varices into small, medium, and large, in addition to including the Child-Pugh cirrhosis grade and the presence of high-risk red spots in the varices. This system demonstrated high specificity for predicting variceal bleeding, but it was not sensitive and had a low positive predictive value.30 Following the Baveno I Consensus in 1992,31 the classification of varices into small (< 5 mm) or large (> 5 mm) was recommended, with this being the best cut-off point for defining the 2 sizes.32 Child-Pugh class C patients with large varices and red spots were shown to have the greatest risk for bleeding within the year following endoscopy.33,34
Once varices have developed, there is an advance from small to large in 5 to 18% (mean 12%) of patients per year,15,35 particularly in those presenting with progressive hepatic disease. The Child-Pugh score has been shown to systematically have an influence on the progression of EV, but the advance of hepatic disease and consequently that of portal hypertension appear to be the most important factors.15,36,37
The incidence of first bleed is variable; in patients without varices the risk is approximately 2% per year, reaching 5% per year in those patients with small varices, and up to 15% per year in patients that develop medium to large varices.12 Therefore, probability is variable, but it can be estimated according to certain risk indicators (variceal size, Child-Pugh class, cherry-red spots).
One study found a significantly higher HVPG in those patients that developed variceal bleeding (20.4 ± 5.1 vs. 16 ± 5.2; p < 0.001).38 The HVPG was above 12 mmHg in all the patients with varices, as well as in those with variceal bleeding. There was a close correlation between the pressure gradient, the presence of varices, and the probability of bleeding. Likewise, it has been found that patients with a HVPG < 10 mmHg have a 90% probability of remaining compensated after a median 4-year follow-up.39 In addition, for every increase of 1 mmHg in the HVPG, there is an 11% increase in the risk for clinical decompensation. HVPG elevation is presently a very important risk factor for developing varices.
Variceal pressure depends on portal pressure. Many studies, such as the one mentioned above, have shown that variceal bleeding does not occur if the HVPG does not reach a threshold value of 12 mmHg.38,40,41 For this reason, if the HVPG is substantially reduced, there is a marked decrease in the risk for bleeding.40,42 This is important, given that portal hypertension is reversible through pharmacologic treatment that effectively diminishes portal pressure.
Portal pressure gradient measurement
The most commonly used method for measuring portal pressure is through HVPG determination, which is an indirect method. The HVPG is the difference between the wedged hepatic venous pressure and the free hepatic venous pressure. The HVPG has been used to evaluate the presence of portal hypertension since its first description in 195143 and is validated as the best predictor for the development of portal hypertension complications.
HVPG measurement consists of placing a balloon probe in a large hepatic vein under radiologic control; once it is in the correct position, it is inflated until it blocks the flow, obtaining the wedged pressure; upon deflation, the free pressure is obtained. This balloon occlusion technique forms a column of blocked fluid from the hepatic vein to the hepatic sinusoids in a broad segment of the liver; it is an easy and rapid technique that has become the standard procedure since 1979.44 Moreover, in experienced hands, HVPG measurement is highly reproducible, precise, and safe.
HVPG measurement has been proposed for the following indications:
1) to monitor portal pressure in patients that are taking drugs to prevent variceal hemorrhage;
2) as an outcome marker39; and
3) in studies that evaluate the pharmacologic agents for treating portal hypertension.45
Portal hypertension gastropathy
The endoscopic diagnosis of portal hypertensive gastropathy (PHG) is based on the presence of a distinctive mosaic-type pattern in the mucosa. This pattern is characterized by small polygonal areas with a depressed edge. There can be red punctiform lesions superimposed on this pattern that are usually larger than 2 mm in diameter. PHG is regarded as mild when there is only a mosaic pattern, and as severe when this pattern is superimposed with red spots.46 The cause and pathogenesis of this PHG are not well understood and its development is correlated with the duration of cirrhosis, but not necessarily with the grade of hepatic dysfunction.
Screening for esophageal varices
Certain noninvasive tests have been shown to be useful in the selection of patients with high risk for having EV, particularly platelet count, splenomegaly,47 data obtained through abdominal ultrasound (portal vein diameter > 13 mm), and recently, the FibroScan.48 However, none of these, individually or combined, are sufficiently precise for definitively ruling out the presence of large EV.49
At a symposium of the American Association for the Study of Liver Diseases,50 it was suggested that cirrhotic Child-Pugh class A patients would benefit from endoscopy when there were data of portal hypertension (platelets < 140,000; portal vein diameter > 13 mm; and ultrasound showing collateral circulation). Child-Pugh B and C patients should undergo endoscopy at the time of diagnosis. Patients without varices should have upper endoscopy every 2 to 3 years if hepatic function is stable, and once a year in the case of signs of deterioration. Due to the fact that EV form when there is an increase in portal pressure above 10-12 mmHg51 and that the development of large varices is more rapid when they are present in the initial endoscopy, the interval should be reduced to every year in patients with small varices and with clinical signs of deterioration (development of ascites and/or hepatic encephalopathy).
Recommendations:
• All patients with hepatic cirrhosis should be evaluated through upper endoscopy; the varices should be classified as small (under 5 mm) or large (≥ 5 mm). (Level of agreement 9).
• Cirrhotic patients without varices should have control upper endoscopy every 2 to 3 years to evaluate the appearance and/or progression in size. (Level of agreement 9).
• In compensated cirrhotic patients with small varices, control upper endoscopy should be carried out every 2 years and every year in those patients with signs of deterioration, in order to evaluate size progression. (Level of agreement 9).
Therapeutic options in patients with portal hypertension
Preprimary prophylaxis
Preprimary prophylaxis is the term used for the prevention of the formation of varices. Experimental studies have suggested there is a benefit from using nonselective beta blockers (NSBBs) for preventing the formation of collaterals.52,53 NSBBs reduce the total portal pressure by 15 to 20%, regardless of liver function and the severity of portal hypertension, or the systemic hemodynamic parameters.54
A study that included 213 cirrhotic patients with portal hypertension, but without varices,10 comparing timolol vs. placebo for a median of 55 months, showed no benefit with the use of NSBBs. There was no difference in the development of EV or their bleeding, and there was also the same frequency of complications (e.g. ascites, encephalopathy, or death). Adverse effects were more frequent in the timolol group. On the other hand, the study showed that a baseline HVPG under 10 mmHg, or a baseline reduction of more than 10%, or a HVPG reduction under 10 mmHg, were the only independent predictors for remaining free from EV. This HVPG reduction was obtained more frequently with timolol and the result was statistically significant.
The low effectiveness of the NSBBs in preventing the formation of varices and the high frequency of adverse effects seen in compensated patients, calls into question the use of NSBBs without endoscopic screening in the search for varices.10
A different available approach is to prevent the progression of cirrhosis, according to its specific etiology, referring the patients to specialized centers.
Recommendation:
• NSBBs are not useful in preventing the formation of varices in patients with portal hypertension. (Level of agreement 9).
Primary prophylaxis
In the past, prophylactic treatment for variceal bleeding was only contemplated in patients with medium to large EV. This was due to the fact that the majority of studies with adrenergic beta blockers were done on this type of patient, whereas the benefit was less clear in patients with small varices.55 However, it has been well established that small varices with red signs, or in patients with Child-Pugh class C, have a risk for bleeding similar to the large varices.33 A controlled study evaluated the role of NSBBs in preventing the growth and bleeding of small varices. This study14 was conducted on 161 cirrhotic patients with small varices, and showed a reduction in the rate of variceal growth in patients receiving nadolol compared with placebo. Additionally, the risk for bleeding at the end of the follow-up was significantly lower in the nadolol group (12%) compared with the placebo group (22%). Based on this, the latest consensus of the Baveno conference concluded that prophylactic treatment with NSBBs could be considered in patients with small EV (without associated risk factors for bleeding) for the primary purpose of reducing variceal growth.7 But further studies are needed to establish this suggestion as a formal recommendation.
Recommendations:
• There is no conclusive evidence on the benefit of NSBB use in primary prophylaxis in the presence of small varices without signs of risk and initial liver failure or in Child-Pugh class A. (Level of agreement 9).
• Patients with small varices and high risk endoscopic signs (red spots) or with advanced Child-Pugh class B and C liver failure should benefit from NSBB use. (Level of agreement 9).
Pharmacologic prophylaxis is directed at preventing the first bleed and improving survival by reducing the mortality related to bleeding. NSBB effectiveness in the prevention of the first bleed has been compared with placebo in 11 randomized controlled studies (RCSs). A meta-analysis of these studies showed a reduction in variceal first bleed risk (from 24% without treatment to 15% with NSBBs after a 2-year follow-up).55 The mortality rate was also lower in the NSBB group and this difference was statistically significant.
Recommendation:
• Primary prophylaxis in the presence of large EV in patients with no contraindications should be initiated with NSBBs. (Level of agreement 9).
It is important to point out that NSBBs are among the least expensive and the safest drugs. They reduce portal pressure by decreasing cardiac output (β-1 effect) and by producing splanchnic vasoconstriction and reducing the portal blood flow (β-2 effect).56 Propranolol and nadolol are the 2 most widely used NSBBs.55 Nadolol is easier to administer due to its longer half-life, enabling once-a-day dosing. Furthermore, it has less liposolubility, it does not cross the blood-brain barrier and thus has fewer side effects on the central nervous system.57 Propranolol is commonly begun at a dose of 20 mg twice a day, while nadolol is started at 40 mg per day. Some studies suggest beginning with nadolol at 20 mg per day and increasing to the maximum dose tolerated with no side effects or to 240 mg.58 As mentioned before, reducing the HVPG to < 12 mmHg essentially eliminates the risk for bleeding and improves survival,40 whereas reductions > 20% of the baseline25 significantly reduce the risk for first variceal bleed. Because the HVPG is not widely available, the NSBB dose should be titrated to reduce the heart rate 25% from the baseline or to 55 bpm. Given that the decrease in heart rate is not correlated with HVPG reduction,56 the NSBB dose should be adjusted to the maximum tolerated dose or until the previously mentioned objectives are reached (heart rate or beats per minute), whichever occurs first. The dose will then be gradually increased until reaching the highest limit of 160 mg twice a day of propranolol or 240 mg once a day of nadolol.
The most common adverse effects are headache, fatigue, dyspnea, impotence, and sleep disturbances. Although they are not usually severe, a dose reduction may be needed; they can also cause poor treatment adherence. Approximately 10-15% of the side effects cause the treatment to be discontinued.59 In addition, NSBB use is contraindicated in close to 15% of patients.60 Absolute contraindications include heart failure, severe obstructive pulmonary disease, second or third-degree heart block, severe aortic stenosis, or peripheral vascular insufficiency. Recently in a prospective study, NSBB benefit was compared between patients with and without refractory ascites61; groups without significant differences were included (including HVPG 20 ± 4.5 vs. 19.1 ± 5). Surprisingly, there was worse survival in cirrhotic patients with refractory ascites. The patients with refractory ascites and NSBB had a shorter median survival (5 months vs. 20 months, p = < 0.0001) and the survival rate at one and 2 years was lower (19 vs. 64% and 9 vs. 45%, p = < 0.0001, respectively). The independent factors that predicted mortality were hepatocellular carcinoma, Child-Pugh class C, etiology of the refractory ascites (kidney failure and hyponatremia), and NSBB use. It is therefore suggested that these drugs not be used in cases of refractory ascites. Finally, an important reduction in cardiac output was demonstrated in patients with hepatorenal syndrome, indicating progression of the circulatory dysfunction in cirrhosis.62 This was determined by a diminished preload and chronotropic function, and as was observed in other studies, a probable cardiomyopathy with left ventricular dysfunction due to cirrhosis.63-65 This cardiac failure is only present in the advanced stages of liver dysfunction, with important renal perfusion alteration, and there is great controversy as to NSBB use in this clinical setting.66
In close to 25% of cirrhotic patients with medium or large EV, NSBB use is contraindicated or they are not tolerated by them, and the degree of protection reached (an approximate 40% relative risk [RR] reduction) is far from ideal. Nitrates diminish portal pressure principally through a reduction in the intrahepatic and portosystemic resistances,35 but they have a systemic hypotension effect and the decrease in portal pressure could be due more to the hypotension than to the reduced resistances.67 Isosorbide mononitrate (ISMN) is the only drug in its class that has been evaluated for the prevention of variceal bleeding. It is ineffective if administered alone60 and could increase morbidity, especially in patients with advanced cirrhosis and ascites.68
The NSBB and ISMN combination has been shown to significantly intensify the long-term response of NSBBs in the HVPG.69 In a randomized multicenter study, the long-term (up to 7 years) use of nadolol plus ISMN was significantly more effective in reducing the first episode of bleeding and with only a few side effects, compared with the use of nadolol, alone.70,71 In contrast to these results, in another study conducted on 349 patients,59 the accumulated probability of the first variceal bleed with the use of propranolol plus ISMN was similar to the propranolol group plus placebo.
In a study of this combination72 conducted on 56 cirrhotic patients with high risk EV (large with red signs) , when NSBBs were used alone at a dose that diminished the heart rate to 55 bpm, there was a therapeutic response in the HVPG in 38% of the patients. When ISMN was added to the NSBBs in non-responders, the overall HVPG response increased to 48% of the patients. This approximation based on strict and early HVPG surveillance, with the sequential addition of ISMN in the patients that did not respond to the NSBB, was able to adequately categorize the patients that were at risk for bleeding. At 2 years, the probability of first variceal bleed in responders was 4%, increasing in non-responders to 22 to 24%. The addition of ISMN caused adverse effects in 17% of the patients, but they were only mild and were eliminated when the dose was reduced, so the combination used in this study was safe. This suggests that the combination of NSBBs and ISMN is safe and effective in primary prophylaxis. However, it could increase the morbidity in patients with advanced cirrhosis. There is still not sufficient evidence to recommend the combination, and new RCSs are expected to clarify these contradictory results.
A recent meta-analysis suggests that in cirrhotic patients not adhering to NSBB use, with contraindications, or with poor tolerance to them, endoscopic ligature (EL) of EV appears to be superior in preventing the first variceal bleed.73 In 19 RCSs, the bleeding rate was lower with EL (odds ratio: OR 0.48; 95% confidence interval [95% CI]: 0.36-0.65; p < 0.0001); when only high quality studies were taken into account this benefit was not maintained and the general mortality, or that associated with bleeding, was not reduced. Moreover, the NSBBs were related to a greater number of adverse events (OR 2.61; 95% CI: 1.6-4.4; p < 0.0001). Current evidence cannot recommend EL as a first-line therapy over the NSBBs. These drugs continue to be valid due to their accessibility and cost. However, EL is a reasonable alternative in centers with experience and in patients with the abovementioned characteristics.
Recommendations:
• The reduction of the HVPG to values under 12 mmHg or a 20% decrease with respect to the baseline value reduces the risk for hemorrhage due to EV. (Level of agreement 9).
• There is no data to support the use of ISMN as mono-therapy in primar y prophylaxis. (Level of agreement 9).
• There is not sufficient evidence for recommending the combination of NSBBs plus ISMN in primary prophylaxis. (Level of agreement 9).
Preprimary and primary prophylaxis in gastric varices
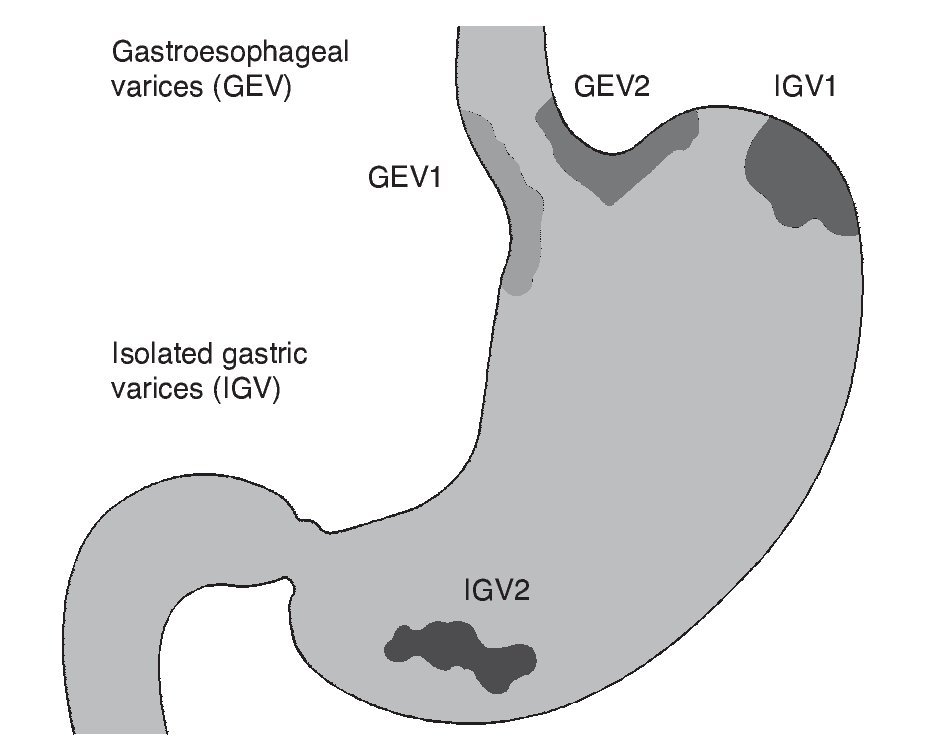
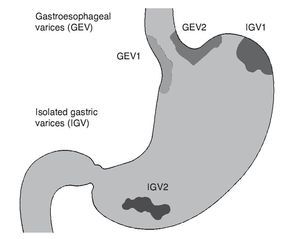
The prevalence of gastric varices (GV) in patients with portal hypertension varies from 18 to 70%.74,75 The incidence of bleeding due to GV is relatively infrequent, from 10 to 36%,75 but mortality from the first bleed due to GV remains at 38 to 55% at 6 weeks.76,77 Generally, GV accompany EV, but they can present on their own. Different classifications have been created, depending on the location of the varices76,78; the distinct subtypes have different natural histories and treatment responses.76,79 GV are subdivided into gastroesophageal varices (GEV) and isolated gastric varices (IGV), as described by Sarin.75 Type 1 GEV (GEV1) are the continuation of EV that extend 2 to 5 cm below the gastroesophageal junction, along the lesser curvature of the stomach. Type 2 GEV (GEV2) extend below the gastroesophageal junction towards the gastric fundus. The IGV are divided into type 1 IGV (IGV1), located in the fundus, and type 2 IGV (IGV2), located in any other part of the stomach (Fig. 1). GEV1 are the most frequent and regularly disappear spontaneously with the obliteration of EV. GEV2 are less common, but are associated with a higher incidence of bleeding and it is less likely for them to disappear with the obliteration of EV. The IGV that do not connect with EV usually occur in the fundus and are more difficult to treat endoscopically.80
Figure 1. Gastric varices classification. Sarin classification: GEV1 are a continuation of the esophageal varices extending up to 5 cm below the gastroesophageal junction along the lesser curvature of the stomach; GEV2 extend below the gastroesophageal junction toward the gastric fundus. IGV are divided into IGV1 located in the fundus and IGV2 located in any other part of the stomach. GEV: Gastroesophageal varices; GEV1: type 1 gastroesophageal varices; GEV2: type 2 gastroesophageal varices; IGV: Isolated gastric varices; IGV1: type 1 isolated gastric varices; IGV2: type 2 isolated gastric varices.
Fundal IGV may result from splenic vein thrombosis, which can be verified through noninvasive imaging studies. These patients frequently require splenectomy for adequate control and decompression of the varices.
Unlike EV, GV present unique difficulties. Traditional endoscopic therapies with EL or endoscopic sclerotherapy (ES) have proved to be significantly less effective in the acute control and prophylaxis of gastric varices.77,81 The increased severity of GV is probably associated with their distinctive anatomy and physiology, particularly with the fundal varices. Anatomically, varices of the gastric fundus are associated with high flow veins arising from gastrorenal, gastrophrenic, or cardiophrenic shunts, which can have a potential for more severe82 and more frequent bleeding at 2 years (55% GEV2 and 78% IGV1).76
At present, there is no data on preprimary prophylaxis in GV and there is only one study evaluating the primary prophylaxis of bleeding due to GV. The role of n-butyl-2-cyanoacrylate (NBCA) was analyzed in an open RCS conducted on 89 patients83 that compared NBCA with NSBB or no treatment, with a follow-up of 26 months. There was bleeding in only 10% of the patients with NBCA, compared with 38% and 53% (NSBB and no treatment, respectively). There was a significant difference between NBCA and the other 2 treatments (p = 0.001, p = 0.003), and this effect was not observed between the NSBB and no treatment groups (p = 0.575). Greater effectiveness was seen with NBCA in the prevention of variceal first bleed in high risk GV (size > 20 mm, MELD > 17 and the presence of portal hypertensive gastropathy) and there was reduced mortality with NBCA, compared with the no treatment patients (7 vs. 26%, p = 0.048). NSBB use in GV did not lower these outcomes despite a HVPG reduction. In this group of patients, the factors that predicted a greater risk for GV bleeding were: size > 20 mm, the presence of PHG, and MELD > 17. In conclusion, there is little information on prophylactic treatment in high risk patients with GV. Based on this study, attention would focus on the use of NBCA with adequate safety and effectiveness. However, due to the absence of specific data on primary prophylaxis in gastric varices, NSBB use is recommended.7
Second module. Acute variceal hemorrhage: initial management, transfusions, antibiotics and pharmacologic treatment
Variceal hemorrhage is one of the most serious complications in patients with cirrhosis, particularly in those that have developed clinical decompensation (ascites, encephalopathy, previous hemorrhagic episode, or jaundice). Bleeding in this clinical setting is more frequently caused by EV (65-75%) or by GV (10-15%).3,84 Mortality at 6 weeks with each episode of variceal hemorrhage is from 15 to 20% and goes from 0% in patients with Child-Pugh class A to 30% in patients with Child-Pugh class C.85,86
Natural history and outcome of acute variceal hemorrhage
Clinical studies show that the bleeding episode spontaneously remits in 40 to 50% of the patients.55 With today's available treatments, bleeding is controlled in more than 80% of the patients.3
Re-bleeding incidence is from 30 to 40% in the first 6 months; the greatest risk presents in the first 5 days, and goes down to the baseline risk after 6 weeks.1 There are treatment failure predictors in the first 5 days such as the presence of bacterial infection,87,88 active bleeding in urgent endoscopy,3,87,89 the presence of portal vein thrombosis,3 and HVPG > 20 mmHg measured shortly after hospital admission89-91; some of them have modified the treatment approach, fortunately managing to reduce the rebleed rate in the first 6 weeks to 20%.3,91,92 This is of interest because early rebleed and sepsis are the 2 most important predictors of death by variceal bleeding.93
Immediate death from uncontrolled bleeding varies from 4 to 8%.3,12 Approximately 60% of the deaths are related to liver failure, infection, or hepatorenal syndrome.3 The consensus is that any death that occurs within the 6 weeks following hospitalization for variceal bleeding should be regarded as a death related to bleeding.94
Frequently reported indicators that increase the risk for death are: the Child-Pugh classification, blood urea nitrogen or creatinine, active bleeding in the endoscopy, hypovolemic shock, and hepatocellular carcinoma.1,3,88,95
MELD significantly predicts mortality in patients with cirrhosis and variceal bleeding. In a study, mortality at 6 weeks was 8% in the patients with a MELD that was less than 18 and 46% in those with a MELD greater than 18.96 Moreover, patients with a high MELD (> 18) and active bleeding had a 10 times higher risk for death within the 6 weeks after variceal bleeding.
Therefore, the outcome for patients with acute variceal bleeding is determined by portal pressure90 and clinical factors, such as the severity of liver disease, the magnitude of bleeding, and biochemical status.
Treatment
Variceal bleed management continues to be a clinical challenge due to its high mortality. Acute variceal bleeding should be managed in an intensive care unit by an experienced medical team that includes well-trained nurses, clinical hepatologists, gastroenterologists, endoscopists, interventional radiologists, and surgeons. The lack of these facilities should be specifically made up for through adequate communication of each institution's multidisciplinary team.
General management
Cautious correction of hypovolemic shock should be started, directing an important part of our management towards preventing the complications responsible for the considerable mortality rate (bacterial infections, hepatic decompensation, and renal failure).
The ABCs (airway, breathing, circulation) are the first step, maintaining adequate oxygen saturation, hemodynamic status, and hemoglobin. In the patient with encephalopathy and important bleeding (hypovolemic shock, bright red hematemesis), the airway must be immediately protected. This is a risk that potentially can be exacerbated by sedation during the endoscopic procedure and so it is recommended to monitor the patient through pulse oximetry and consider intubation in the patient with important bleeding.
These patients should be carefully managed in relation to resuscitation with fluids, blood, or volume expanders. Prolonged hypotension should be avoided in order to prevent infection, renal failure, and liver function deterioration, which are associated with increased risk for rebleeding and death.95 Despite the fact that with volume expansion there can be an increase rebound in portal pressure, and secondarily in rebleeding,97,98 the use of vasoactive agents decreases the magnitude of the increase in portal pressure.99,100 Hemoglobin of 7 to 8 g/dL94 is recommended, with higher figures in patients with cardiopathy or active bleeding. Overtransfusion should be avoided because it can result in an increase of portal pressure with the consequent increased risk for early rebleeding, as well as pulmonary congestion.101,102
The placement of a nasogastric catheter and gastric content aspiration are common practices, but no improvement has ever been documented in relation to survival or a reduction in complications, and so their use is still controversial.
Initial treatment for acute variceal bleeding is based on the combination of vasoactive drugs with endoscopic therapy. Diagnostic endoscopy should be done as soon as possible after hospital admission (within the first 12 h), especially in patients with clinically significant bleeding. This recommendation, based on clinical guides from different countries, is adopted from expert opinion.7,103,104 A longer delay (up to 24 h) may be acceptable in cases of mild bleeding (stable patients with a systolic pressure > 100 mmHg and heart rate < 125 bpm) with complete response to vasoconstrictors or if the endoscopy equipment or an endoscopist are not immediately available.105,106
Recommendations:
• In all cirrhotic patients with upper gastrointestinal hemorrhage of probable variceal origin (hematemesis and/or melena), resuscitation methods (vascular approach and volume replacement), airway protection (considering intubation in the patient with important bleeding and encephalopathy), and vasoactive drug administration should be started as soon as possible - even before carrying out endoscopic study. (Level of agreement 8).
• All cirrhotic patients suspected of having acute variceal hemorrhage should have upper endoscopy within the first 12 h of hospital admission. (Level of agreement 9).
• When there are EV and there are no other lesions that explain the hemorrhage, bleeding is considered to be of variceal origin, and the patient is offered the corresponding therapeutic option. (Level of agreement 9).
• There is no evidence in regard to the usefulness of nasogastric catheter placement. (Level of agreement 9).
Complication treatment and prevention: infection prevention
Bacterial infections are present in 20% of the cirrhotic patients with upper gastrointestinal bleeding upon hospital admission and another 50% are at risk for becoming infected.107-111 Spontaneous bacterial peritonitis, urinar y tract infection, and pneumonia should be looked for and ruled out, due to their high prevalence. In this group of patients, antibiotics reduce rebleeding109 and mortality,111 and therefore their use is recommended from the moment there is suspicion and/or diagnosis of acute variceal bleeding. Two meta-analyses108,111 showed that the short, prophylactic use of antibiotics has a beneficial effect on mortality, with an approximate 9% reduction.
Quinolones are frequently used because of their easy administration, good oral absorption, and low cost.112 Quinolone (norfloxacin) doses of 400-500 mg twice a day for 5 to 7 days are used. A reduced RR for mortality of 29% (95% CI, 6-46%) was observed, as well as a 58% (95% CI, 48-66%) reduction in infection incidence in patients that received antibiotic prophylaxis, compared with placebo.
Intravenous ceftriaxone (1 g per day) has recently been shown to be superior to oral norfloxacin (400 mg twice a day) in high risk patients (hypovolemic shock, ascites, jaundice, and malnutrition) for reducing the development of infections (33 vs. 11%, p = 0.03).110
And finally, aminoglycoside use should be avoided due to the high risk for nephrotoxicity.113
Recommendation:
• Patients with acute variceal hemorrhage should receive antibiotics such as oral norfloxacin 400 mg every 12 h or parenteral ceftriaxone 1 g per day in high risk patients (hypovolemic shock, ascites, jaundice, and malnutrition) upon hospital admission and for a period of 5 days. (Level of agreement 9).
Specific treatment of variceal hemorrhage in portal hypertension
Vasoactive drugs
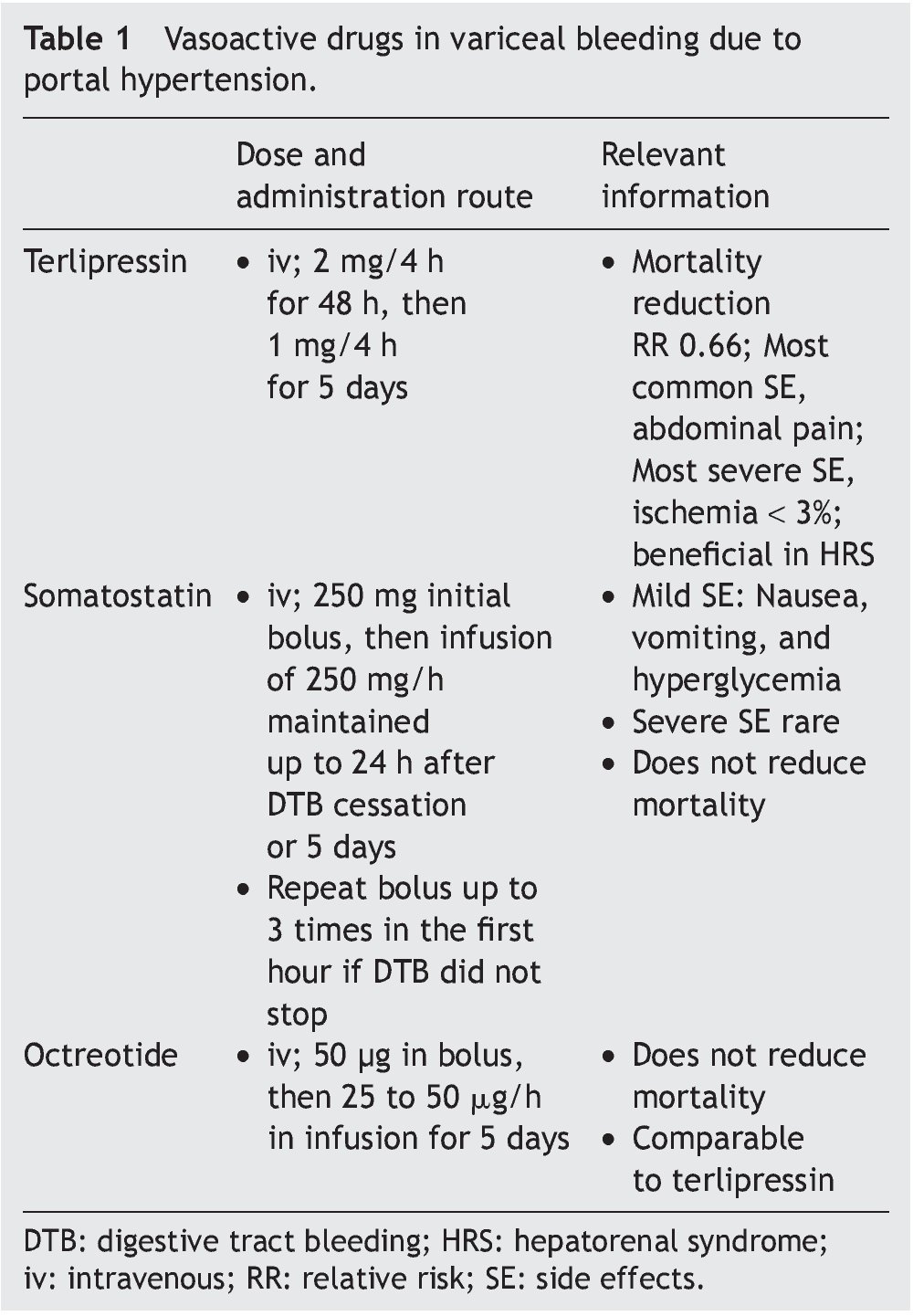
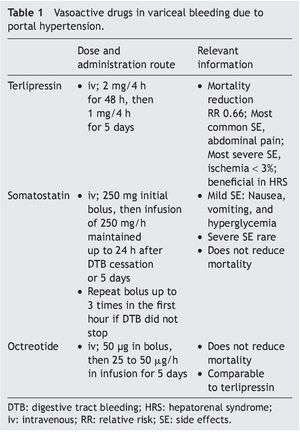
The aim of pharmacologic treatment is to reduce portal pressure, which is closely correlated with variceal pressure. This was observed in initial studies90,114 that showed that a portal pressure above 20 mmHg was associated with worse outcome, and was recently confirmed in another study that used somatostatin99 to reduce portal pressure during an episode of acute variceal hemorrhage, improving outcome. Drug selection is dependent on local resources. Terlipressin should be the first choice because it is the only drug that has demonstrated survival improvement.55,115 These drugs are easily administered and quite safe. Treatment can begin, even during patient transfer, which can increase survival in patients with massive bleeding; furthermore, these drugs can facilitate the endoscopic procedure.115 Two different types of drugs with distinct action mechanisms are used: vasopressin and its analog, terlipressin, and somatostatin or its analogs (Table 1).
Terlipressin. A long-acting drug derived from vasopressin, terlipressin is a triglycil lysine. It has a lower number of side effects, mainly abdominal pain. Severe adverse effects such as peripheral or myocardial ischemia occur in less than 3% of the patients.116 Terlipressin reduces cardiac output and increases arterial pressure and systemic vascular resistances, leading to a decrease in splanchnic vascular affluence. This reduction, added to the vasoconstriction of the splanchnic vasculature diminishes portal pressure by approximately 20% after the first dose.117 The effect is obtained within the first 30 min and remains significant up to 4 h after administration. When variceal bleeding is suspected, a dose of 2 mg every 4 h for the first 48 h is recommended, followed by a reduced dose of 1 mg every 4 h for up to 5 days.116
Terlipressin significantly improves bleeding control and survival118 and is the only drug that has demonstrated improvement in the outcome of variceal bleeding in RCSs.55,118 Recent meta-analyses indicate that terlipressin is associated with a statistically significant reduction in any cause of death, compared with placebo (RR 0.66, 95% CI, 0.49-0.88). And lastly, it has an overall effectiveness in bleeding control of 75 to 80% at 48 h,115 of 67% at 5 days,116 and a beneficial effect on renal function in patients with decompensated cirrhosis.95
Somatostatin and its analogs. Somatostatin. Through experience of more than 3 decades, it is known that high doses (500 mg/h) have a pronounced effect on the HVPG, with greater clinical effectiveness in the subgroup of patients with active bleeding during emergency endoscopy.119 Treatment should be initiated with a bolus of 250 mg, followed by an infusion at 250 mg/h until reaching a period of 24 h free-from-bleeding, or maintaining treatment up to 5 days to prevent rebleeding.120 The initial bolus can be repeated up to 3 times during the first hour if bleeding is persistent. The side effects are mild; nausea, vomiting, and hyperglycemia occur in 30% of the patients.119-121 Despite its beneficial effect on the control of bleeding, somatostatin has no impact on mortality.55
Octreotide. An analog of somatostatin, this drug has a longer half-life, although it does not have a longer hemodynamic effect.122 The administration of an initial 50 μg bolus is recommended, followed by an infusion at a dose of 25 or 50 μg/h123; likewise, it can be maintained for 5 days to prevent early rebleeding. Its safety profile is similar to that of somatostatin and its effect is comparable to terlipressin. However, none of the studies has been double-blinded and so they lack strength.55 The statistically significant reduction of early rebleeding with the use of sclerotherapy plus octreotide could be due to the prevention of the postprandial increase in portal pressure123,124; similarly, it does not modify mortality.55,124
Recommendations:
• In cases of acute hemorrhage of variceal origin, the administration of vasoactive drugs should be continued for 3 to 5 days, to cover the period of maximum risk for rebleeding. (Level of agreement 9).
• Of the different pharmacologic management options during the acute episode of variceal hemorrhage, terlipressin is the only vasoactive agent that has been shown to reduce mortality. (Level of agreement 9).
Endoscopic therapy
ES consists of the intravariceal or paravariceal injection of a sclerosing agent. It is carried out every 10 to 14 days until the varices are eradicated, which takes an approximate 5 to 6 sessions. In EL, the varices are strangled through the application of elastic bands on the varicose vein, usually placing 5 to 8 bands per session. EL of the EV is done every 2 to 3 weeks until the varices are obliterated or until they can no longer be ligated, generally in 3 to 4 sessions. However, due to the fact that the rebleed rate can be as high as 50% after ES,125 this procedure has been almost universally replaced by EL. A meta-analysis of 7 studies that comprehended 273 patients,126 showed a significant 50% rebleed reduction with EL, including variceal rebleeding and that induced by ulcers.
Endoscopic therapy is widely recommended in all patients presenting with acute variceal bleeding. ES has been shown to be effective in the control of acute bleeding and in preventing rebleed, compared with medical treatment with vasopressin or balloon tamponade.127 However, endoscopic treatment requires a qualified endoscopist; in particular ES is frequently associated with adverse events.126,128 EL has been compared with sclerotherapy in various RCSs and in one meta-analysis in relation to the long-term prevention of variceal bleeding, and it was found to be superior to sclerotherapy.126 In this meta-analysis, EL reduced the rebleed rate (OR 0.52; 95% CI 0.37-0.74), mortality (OR 0.67; 95% CI 0.46-0.98), and the rate of death associated with bleeding (OR 0.49; 95% CI 0.24-0.99) when compared with ES. With a limited number of ELs, a positive effect was achieved in regard to the prevention of episodes of rebleeding (4 ELs in the place of ES prevent one rebleed episode) and death (10 ELs instead of ES to prevent one death). EL has fewer complications, does not increase portal pressure (compared with sclerotherapy),129 and requires fewer procedures to eradicate EV.85,125,130 In cases of severe and profuse bleeding, EL can be technically difficult because the blood reduces the visual field; only in such cases could sclerotherapy be the initial treatment. For all these reasons, EL should be the endoscopic treatment of choice in acute variceal bleeding.
Recommendations:
• Endoscopic treatment options (ES and/or EL) are useful in managing the acute episode of variceal hemorrhage. (Level of agreement 9).
• EL is the first choice in the endoscopic management of the acute hemorrhagic episode of EV. (Level of agreement 9).
• Variceal EL has a lower complication rate than ES. (Level of agreement 9).
Current recommendations for initial management
The present recommendation is to begin with pharmacologic treatment (ideally in the transfer to the hospital, even if only a variceal origin is suspected) as early as possible and perform EL (or ES if ligature is technically difficult) after initial resuscitation. This is based on RCSs that have shown that early initiation with vasoactive drugs facilitates endoscopy and improves the control of the bleeding and rebleeding of the first 5 days.115,121,131,132 A meta-analysis of 8 randomized studies133 evaluated the combination of endoscopic and pharmacologic treatment against endoscopic therapy alone in the control of acute variceal bleeding and found that there was improvement in the initial control of bleeding (RR 1.12; 95% CI 1.02-1.23) and in the hemostasis of the first 5 days (RR 1.28; 95% CI 1.18-1.39) with a number needed to treat of 8 and 5, respectively. This improvement was obtained with no increase in adverse effects; the benefit remained significant in the studies that had a low proportion of alcoholics (< 40%) or that excluded high risk cirrhotic patients (< 35%). However, mortality was not significantly reduced (RR 0.73; 95% CI 0.45-1.18).
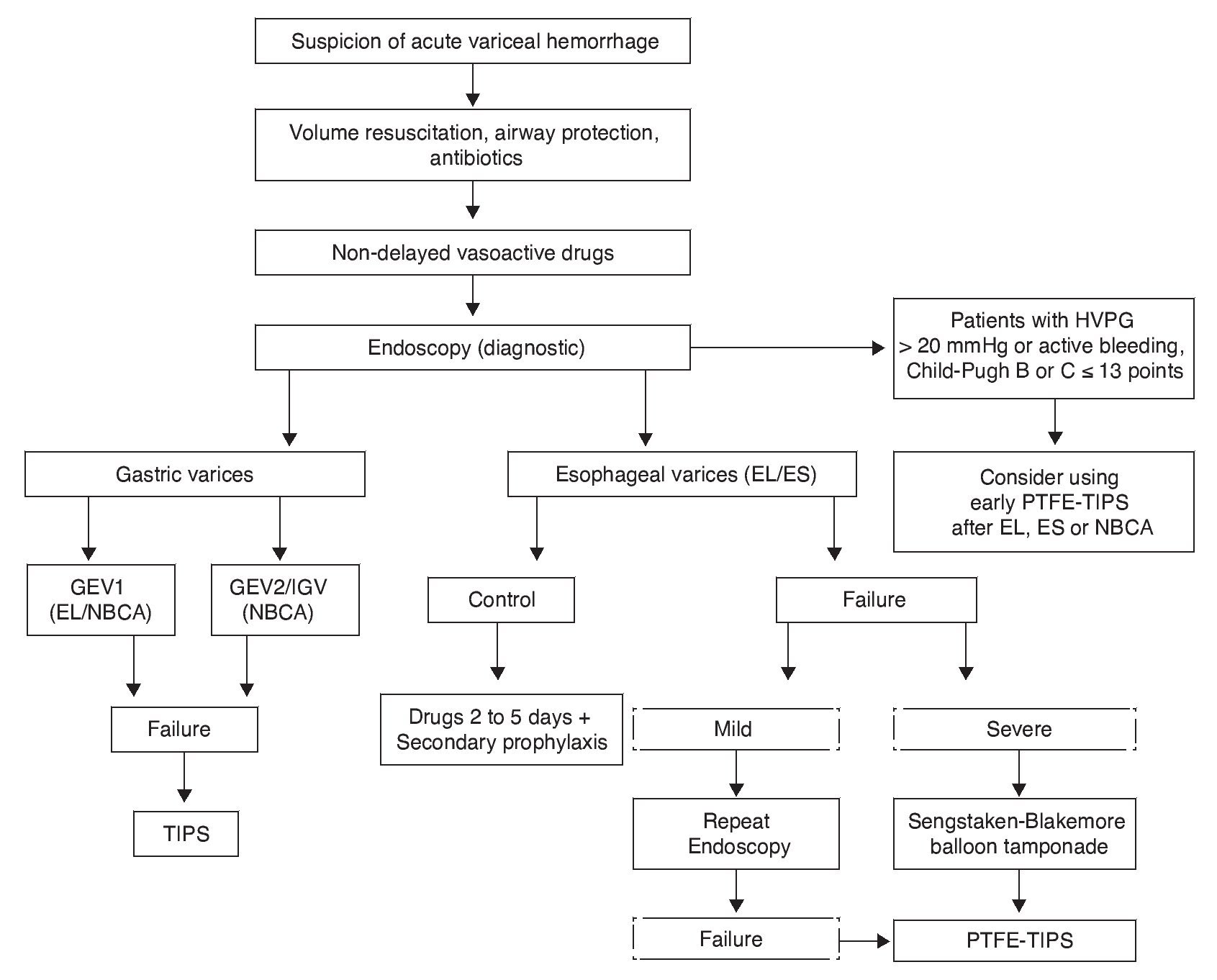
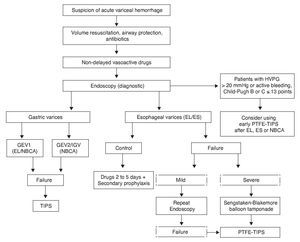
Endoscopic therapy and vasoactive treatment have demonstrated effectiveness in the control of bleeding in 80 to 85% of the patients. Pharmacologic treatment improves the results of endoscopic treatment if it is begun just after ES and EL,55,124 and so the current recommendation is to maintain the drugs for 2 to 5 days to cover the period of greater rebleeding7 Fig. 2).
Figure 2. Acute variceal hemorrhage management. Flow diagram showing acute variceal bleed management. In high-risk patients (Child-Pugh class B or C ≤ 13 points with HVPG > 20 mmHg) early TIPS should be considered. When a patient is suspected of having acute variceal hemorrhage, volume resuscitation must immediately be started, taking care to maintain hemoglobin at around 8 g/ dL, along with judicious crystalloid resuscitation. Airways must be protected in patients with hemodynamic repercussions and encephalopathy. Measures that increase the success rate and prevent rebleed should be stressed, such as prophylactic antibiotics, vasoactive drugs, and endoscopic methods. EL is preferred over ES for esophageal varices, and patients with hemostatic failure have the option of a balloon catheter as a bridge for a more effective and lasting measure, such as TIPS. In patients with variceal bleeding of gastric origin, first-choice treatment is NBCA application, leaving EL only for patients with active bleeding in GEV1 in the absence of NBCA. If there is HVPG measurement > 20 mmHg or active bleeding during diagnostic endoscopy, despite the correct use of a vasoactive drug in patients with Child-Pugh class B or C ≤ 13 points, early PTFE-TIPS (72 h) should be considered after EL, ES, or NBCA. EL: Endoscopic ligature; ES: Endoscopic sclerotherapy; GEV1: Type 1 gastroesophageal varices; HVPG: Hepatic venous pressure gradient; IGV: Isolated gastric varices; NBCA: N-butyl-2-cyanoacrylate; PTFE-TIPS: Transjugular intrahepatic portosystemic shunt with polytetrafluoroethylene-covered stent; Severe Failure: Unstable patient with massive rebleeding.
Recommendation:
• The combined treatment of variceal EL and vasoactive drugs is superior to isolated endoscopic treatment. (Level of agreement 9).
Thrombocytopenia and coagulopathy in the cirrhotic patient
The administration of platelets and fresh frozen plasma has not been adequately evaluated in patients with acute variceal bleeding,134 but it would appear reasonable to maintain platelets between 40,000 and 50,000/mm3 in those patients presenting with acute variceal hemorrhage and thrombocytopenia. In relation to plasma transfusion, recent studies suggest a hypercoagulable state in patients with cirrhosis caused by an imbalance between procoagulant and anticoagulant factors (high factor VIII and low protein C)135; due to the fact that coagulation tests only evaluate procoagulant activity (INR), they could have limitations. When the problems that are implicit in plasma and platelet concentration transfusion are added, such as acute pulmonary damage associated with transfusions and volume overload and infections, its role in the patient with acute variceal hemorrhage becomes questionable. Therefore, its use will continue to be left to the decision of the attending physician until there are well-designed studies that can demonstrate the utility of this measure.
The use of recombinant activated factor VII (rFVIIa), which corrects prothrombin time in cirrhotic patients,136 has been evaluated in 2 RCSs as an adjunct to endoscopic and pharmacologic treatment.137,138 In the first study, no effect on the control of acute bleeding and rebleeding or on mortality was observed. The second study, which only included cirrhotic patients with a Child-Pugh score < 13, showed a significant improvement after rFVIIa administration in the control of bleeding in the first 24 hours, rebleeding between 24 h and 5 days, and death in the first 5 days only in the Child-Pugh class B and C patient subgroup with variceal bleeding (considering at least one of the previous outcomes: 15 failures in 64 patients with placebo compared with 5 failures in 62 patients with rFVIIa [p = 0.03]; specifically in the control of bleeding in the first 24 h: 7 failures in 63 patients with placebo compared with 0 failures in 62 patients with rFVIIa [p = 0.01]). They also showed that rFVIIa use did not increase the number of thrombotic events, and thus appears to be safe to use in this clinical setting. Further studies are required to define the specific population of patients that would benefit from rFVIIa, as well as the minimum effective dose of the drug. Therefore it should only be regarded as rescue therapy when all other treatments have failed.
Recommendations:
• The administration of plasma and platelet concentrations has not been shown to be useful in acute variceal hemorrhage control (even though plasma may have other usefulness). (Level of agreement 8).
• There is no evidence to support the routine use of factor VII. (Level of agreement 8).
Hepatic venous pressure gradient. Its role in hemorrhage secondary to portal hypertension
Its greatest usefulness in clinical practice is in relation to evaluating the hemodynamic response to pharmacologic treatment, for the purpose of analyzing treatment effectiveness and predicting the risk for rebleeding of EV. However, the necessity of having the adequate equipment and reliable operators, as well as its high cost, has discouraged its use outside of the specialized liver units dedicated to treating portal hypertension.
Previous studies on cirrhotic patients admitted for acute variceal hemorrhage have demonstrated that a HVPG above 20 mmHg is associated with an increase in treatment failure rate in up to 50% of the patients.90,91 And so early HVPG measurement was proposed in patients with variceal bleeding in order to select those that would benefit from a more aggressive initial management. In a study on 52 patients,91 those with an initial HVPG above 20 mmHg were randomly distributed to receive conventional treatment or the placement of a transjugular intrahepatic portosystemic shunt (TIPS). The failure rate in patients with conventional treatment was 50% compared with a 12% failure rate in patients with TIPS. A second study86 showed that treatment failure in the first 5 days was 4 times higher in those patients with a HVPG above 20 mmHg (Fig. 2).
Another very interesting study92 demonstrated greater bleeding control and reduced rebleeding with the early use of TIPS, and the probability of remaining free from bleeding at one year was significantly higher in the TIPS group, 97% compared with 50% (p < 0.001); in addition, greater survival at one year (86 vs. 61%) with no increase in adverse events was observed. The main differences from previous studies were the use of early TIPS (within the first 72 h) in high-risk patients (active bleeding during the diagnostic endoscopy despite vasoactive drug administration) and the use of polytetrafluoroethylene (PTFE)-covered stents. In this group of critical patients, the potential adverse effects of the covered TIPS would appear to be balanced by the high effectiveness of bleeding control, preventing progressive clinical deterioration.
It is important to mention that these excellent results were corroborated through a follow-up and surveillance study conducted in the same centers as the initial RCS.139
Therefore, we feel it is of great use to keep in mind the 2 main criteria for using early TIPS: the first is hemodynamic (difficult access), based on a HVPG above 20 mmHg, and the second, endoscopic (easier access), that consists of the presence of active bleeding during the diagnostic endoscopy, despite the correct use of a vasoactive drug.
Recommendations:
• HVPG measurement is recommended in cirrhotic patients in the following settings: uncontrolled variceal hemorrhage, secondary prophylaxis with recurrent variceal bleeding. (Level of agreement 9).
• In the management of patients with acute variceal hemorrhage, HVPG measurement above 20 mmHg identifies patients with a higher risk for rebleeding and death. (Level of agreement 9).
Rescue therapies: balloon tamponade, self-expanding metallic stents, transjugular intrahepatic portosystemic shunt, surgical shunt
In 10-20% of patients, variceal bleeding does not respond to initial endoscopic and/or pharmacologic treatment. If bleeding is mild and the patient is stable, a second endoscopic procedure should be attempted. If this fails or bleeding is severe, diversion treatment should be offered before there is greater deterioration in the clinical status of the patient. Rescue therapies due to treatment failure include balloon tamponade and portosystemic diversions. In cases of massive uncontrolled bleeding, the placement of a Sengstaken-Blakemore balloon tamponade should be considered.
The primary objective of placing a balloon tamponade in the esophagus is to mechanically stop variceal bleeding of the digestive tract. The most commonly used balloon tamponades are the Linton-Nachlas and Sengstaken-Blakemore tubes. Balloon placement manages to temporarily stop the bleeding in 40-90% of the patients.11 However the clinical physician must be aware that with this transitory method there is a high rebleeding recurrence, close to 50%, and an elevated complication rate of infection and/or perforation.140,141 Therefore strict monitoring of the patient is required during the use of this method.
Another mechanical method recently reported on in patients with difficult-to-control variceal hemorrhage is the use of covered self-expanding metallic stents142; they have the advantage over balloon tamponades of presenting with fewer immediate complications.
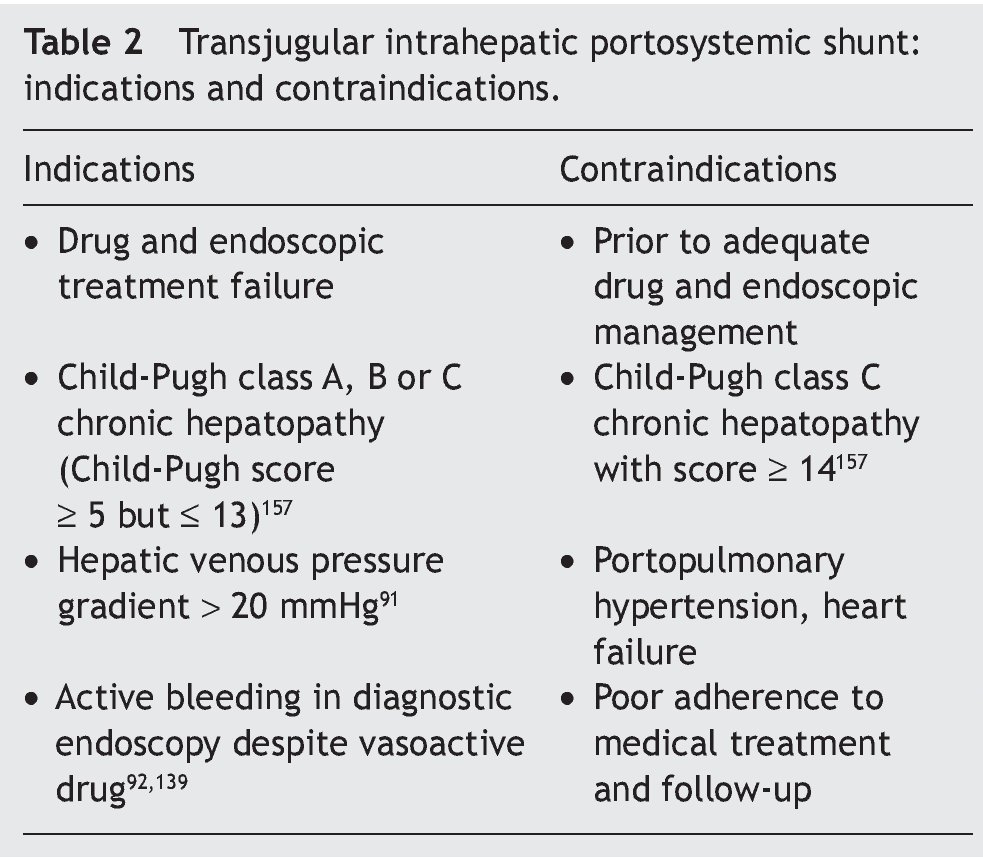
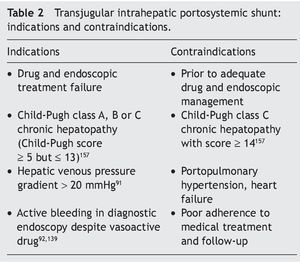
The TIPS consists of the creation of a channel through the hepatic parenchyma, connecting a branch of the portal vein with a branch of the hepatic vein. This communication is achieved through interventionist radiology techniques. The channel is maintained permeable with a self-expandable metallic stent, that ideally is covered.143
This method, like surgical diversion, is effective in variceal bleeding control, with a success rate close to 95%. Due to the fact that TIPS has shown greater efficacy and simplicity, as well as better cost-effectiveness,144 surgical shunts have become the second method of choice.
It has recently been shown that, with the advent of the PTFE-covered stents, long-term permeability improved significantly, the number of clinical relapses decreased, and the number of re-interventions went from 44 to 13% per year,143 with no significant increase in encephalopathy (Table 2).
Despite the excellent hemostatic effect, TIPS placement has not modified the long-term survival of these patients, with a 38% mortality rate at 6 weeks, reflecting the critical clinical status of these patients and the importance of the underlying disease. As mentioned before, recent interest is centered on the early use of this method in the subgroup of patients with greater morbidity and mortality due to rebleeding with a HVPG above 20 mmHg, obtaining a significant reduction in treatment failure and mortality91 (Fig. 2).
TIPS use in the acute hemorrhage secondary to portal hypertension setting is confined to the rescue therapy category, and as such, it should be attempted after pharmacologic and endoscopic treatments have failed. Endoscopic therapy failure is defined as the persistence of bleeding after 2 therapeutic endoscopies for EV and one session for GV.145 In these cases TIPS insertion should not be delayed.
As a consequence of effective non-surgical treatment availability, emergency surgery is no longer the first-choice course of action. The most widely used surgical diversion options used in the past in emergency situations (a heterogeneous definition among studies, presence of active bleeding up to 72 h after initial bleeding) are the non-selective shunts and devascularization methods. However, it is important to clarify that this information comes from small case series and the majority of these methods are compared with sclerotherapy.146-148
Non-selective shunts were the most widely used emergency procedures because of their effectiveness and speed in the control of bleeding, limiting the uncontrolled hemorrhagic event. These are portacaval shunts (end-to-side and side-to-side) and mesocaval shunts, with the insertion of a 16 to 19 mm prosthetic graft. The disadvantages are: a growing number of encephalopathy episodes149 and late occlusion that predisposes rebleeding.150 In patients that are transplantation candidates, a TIPS is the suggested first choice, and when this procedure is not available or is contraindicated, mesocaval shunt is recommended due to the fact that: 1) it eliminates the need for hepatic hilum dissection and 2) it is easily reversible through ligature. In the majority of the studies in which there was a high percentage of Child-Pugh class C patients, mortality rates were from 30 to 41%146,151-153; in one of the studies the mortality rate was 19% because only Child-Pugh class A and B patients were taken into consideration.152
Gastroesophageal devascularization and esophageal transection (modified Sugiura procedure) were widely used,147,148,154,155 and when compared with emergency sclerotherapy, they have a similar early mortality, while in relation to rebleeding control some studies favor transection.147,154,155 There appears to be an inferior control of bleeding, with higher rebleeding rates, but a lower frequency of encephalopathy when compared with the non-selective shunt.148 It is important to remember that it has not been possible to reproduce the large Sugiura case series,156 in which there was a 13% mortality rate and a low 5% of cases of rebleeding.
In relation to patients with bleeding from GV, no statistically valid methods have been used to evaluate the efficacy of surgical methods as rescue treatment. Some studies achieved excellent bleeding control through the use of shunts, with a high incidence of encephalopathy.158 Devascularization was rarely used and presented with a notably high rebleeding rate of up to 40%.
Therefore, surgical treatment should be considered for rescue treatment when the endoscopic and radiologic methods and medical treatment have failed in patients with better liver function, keeping in mind that postoperative morbidity and mortality increases in direct proportion to persistent bleeding duration. Esophageal transection is the appropriate procedure in centers lacking experience in the use of shunts and TIPS. The best surgical candidates are Child-Pugh class A cirrhotic patients. It is important to emphasize that, in contrast, for Child-Pugh class C patients, TIPS is the only applicable rescue therapy because of the high surgical mortality rate in these patients.
Recommendations:
• The use of a Sengstaken-Blakemore balloon is only a temporary measure in the control of upper digestive tract bleeding due to portal hypertension, when conventional treatment has failed, because its use for more than 24 h is associated with different potentially severe complications. (Level of agreement 9).
• Rescue therapies for variceal bleeding are TIPS placement or surgical diversion if the clinical condition of the patient allows for it. (Level of agreement 9).
• In patients with acute esophageal varices bleeding with medical and endoscopic treatment failure, a second endoscopic treatment should be attempted before contemplating other alternatives such as TIPS or surgical diversion. (Level of agreement 9).
• The use of TIPS (preferably covered with PTFE) is recommended in patients with hemorrhage due to EV and/or gastric varices in which other treatments have failed. (Level of agreement 9).
• Surgical diversion is restricted to patients with Child-Pugh class A liver function. (Level of agreement 9).
• TIPS is the only applicable alternative in patients that are not surgical candidates due to an advanced grade of liver failure or to other comorbidities. (Level of agreement 9).
Endoscopic treatment of gastric varices in acute variceal hemorrhage
Of all the acute variceal bleeding events, GV make up approximately 10 to 20% of the episodes in patients with portal hypertension.77,159 Bleeding due to GV tends to be more severe, requiring a greater number of transfusions, and it has a higher mortality rate than bleeding due to EV81; after an acute episode is controlled, GV have a high rebleeding rate of 34 to 89%.76
In the majority of the studies, the patients are treated concomitantly with intravenous terlipressin, wide spectrum antibiotics for 3 to 5 days, and initial resuscitation, showing a similar benefit to that observed in bleeding due to EV. In relation to the specific therapy for gastric varices, there are 2 primary options: endoscopic treatment with the application of tissular adhesives or the radiologic placement of a TIPS. TIPS was the first-line treatment in countries such as the United States, that had limited regulation of tissular adhesives such as NBCA, which is widely used in other parts of the world (Fig. 2).
In 1986 it was first reported that bleeding caused by GV could be controlled through sclerotherapy with the tissular adhesive agent, NBCA.160 NBCA is the most promising of all the agents studied161-167; it immediately polymerizes into a firm coagulate when it comes into contact with blood.168,169 It is injected exclusively within the varicose vein and obliterates large varices. Necrosis of the variceal wall occurs 3 to 4 days later and the NBCA mold slowly detaches after weeks or months. Primary hemostasis of the acute bleeding due to GV varies from 70 to 97% with the use of NBCA, with an early rebleeding rate between 0 and 28% within the first 48 h.76,160,170 As can be observed, the rebleeding rate continues to be high, which is far from ideal. It is possible that the drainage veins and tributaries of the GV, which are especially important in rebleeding reduction,171,172 are not adequately obliterated with the conventional NBCA dose. A decrease in rebleeding has also been demonstrated with repeat injections until complete obliteration (late rebleeding, 18.5 vs. 44.7%), which is easily corroborated when a varix is firm to the touch.173 This therapy improves and controls rebleeding, but the elevated mortality rate primarily reflects advanced liver disease, which is not modified with the use of NBCA.174,175
Thromboembolism has been reported in rare cases and is a serious and catastrophic complication of sclerotherapy with NBCA.159,164,165,168,169,176-179 The risk seems to be related to the size of the varices, their blood flow, the volume of NBCA injected, and the velocity of the injection.169,180 At high doses, fever is frequently observed and is caused by an extensive foreign body reaction, inflammation of the post-sclerotherapy ulcer or bacterial infection. Bacteremia presents in up to 33% of the patients with acute bleeding due to GV after NBCA application.181
Different gastroenterologists have used distinct NBCA doses,170,182 and the ratio of the sclerosing agent dilution to the lipoid agent is different.173,183 The effective dose and the dilution of the sclerosing agents are still the subject of debate. Embolization can occur if an excessive amount of NBCA per application is used or if the adhesive is over-diluted with lipiodol. Therefore, some authors recommend dilutions of 0.5:0.8 to 1:1, with which the polymerization process is delayed (more than 20 s). An over-dilution increases the risk for embolization and application without dilution causes rapid occlusion of the injecting catheter.164,165,168,169,176,178,179,184 The maximum quantity per application must be strictly limited to 1 mL to prevent this complication in gastric fundus varices. If higher doses are required due to the size of the varix, the injections should be applied sequentially.
As was mentioned above, due to the higher hemostasis rate and the lower rebleeding rate, obliteration of GEV2 and IGV with NBCA is used as first-line therapy; this is based on the high percentage of rebleeding with EL.80,165,166,170,178,179
Recommendations:
• EL is recommended for GEV1 but not for GEV2 or IGV1. (Level of agreement 9).
• The added use of vasoactive drugs to endoscopic treatment is recommended in the treatment of acute hemorrhage due to GV. (Level of agreement 9).
• NBCA application is effective in the treatment of hemorrhage due to gastric varices. (Level of agreement 9).
Portal hypertensive gastropathy in acute hemorrhage
PHG causes less than 10% of acute bleeding due to portal hypertension. Small studies have suggested that octreotide can be useful in the control of acute bleeding.185 In the case of important bleeding due to PHG, TIPS has been used in an effort to reduce the need for transfusion.186
Recommendations:
• In those patients with acute hemorrhage secondary to PHG in which conventional treatment has failed, other alternatives such as TIPS or surgical diversion should be employed, depending on the patient's liver reserve. (Level of agreement 9).
• Patients presenting with PHG should be treated with vasoactive drugs. (Level of agreement 9).
Third module: secondary prophylaxis
The risk for variceal rebleeding within the first 2 years is 60%, with a 35% mortality rate.55 Therefore, rebleed prevention is essential in the management of patients with variceal bleeding in order to avoid rebleeding and death. Secondary prophylaxis is initiated after the patient has recovered from the acute episode of variceal hemorrhage, which is usually on the sixth day.7
Recommendation:
• Upon release from the hospital, a patient with variceal bleeding should begin secondary prophylaxis with drugs, endoscopic eradication of varices, or rescue therapies (surgical diversion or TIPS). (Level of agreement 9).
Drug therapy
Assessments of the effect of drug therapy in portal pressure reduction for the prevention of rebleeding in variceal hemorrhage have been carried out. The drugs studied are NSBBs, alone or combined. In addition, other drugs that do not have an effect on portal pressure, such as the proton pump inhibitors (PPIs) and sucralfate, have been used.
Nonselective beta-blockers plus isosorbide mononitrate
A study compared propranolol with propranolol plus ISMN in the prevention of variceal bleeding and found a lower rebleed probability at 2 years of follow-up in the combined therapy group (40.4 vs. 57.4%, p = 0.09), but without reaching statistical significance.187 Likewise, other studies reported a lower rebleed rate, around 33-35%, with the combined therapy when compared with NSBBs alone, but with more adverse effects (fatigue, dyspnea, postural hypotension, fluid retention, and renal failure).9,55,187
Other drugs whose usefulness has been examined in relation to variceal rebleeding prevention are the PPIs and sucralfate. Different studies evaluating the use of sucralfate in the prevention of bleeding after sclerotherapy have shown a rebleed reduction during the eradication of varices through sclerotherapy. This benefit in the ulceration of the mucosa was found only in Child-Pugh class A and B patients, without having an effect on mortality.188,189 Another randomized prospective study on 122 patients with variceal bleeding190 evaluated the use of sucralfate plus nadolol and EL vs. the use of EL alone in relation to rebleed prevention, and found that the triple therapy had less rebleeding (23 vs. 47%, p = 0.005) with a mean 21-month follow-up. In addition, the GEV (12 vs. 29%, p = 0.001) were improved. However, this triple treatment benefit could be largely due to the EL and nadolol.
In relation to the PPIs, a study that compared 40 mg of pantoprazole every 24 h for 10 days with placebo in patients that underwent EL191 reported the same number of post-ligature ulcers in both groups; however, the ulcers in the pantoprazole group were smaller, and 3 patients in the placebo group presented with rebleeding. These results would appear to favor pantoprazole use, but it has to be kept in mind that the study was small and the results have not been reproduced.
Recommendations:
• The combined use of NSBBs plus ISMN is effective, but it must be individualized due to side effects and tolerability. (Level of agreement 9).
• There is not enough evidence to recommend PPI or sucralfate use for the prevention of variceal rebleeding. (Level of agreement 9).
Endoscopic therapies
Therapeutic options consist of ES or EL.
Endoscopic sclerotherapy versus endoscopic ligature
Multiple RCSs comparing EL with ES have shown less rebleeding with EL (RR 0.46; 95% CI 0.35-0.60), fewer complications, and a lower number of endoscopic sessions, with no difference between the procedures in regard to the recurrence of varices or the mortality rate.127,192
The overall rebleed risk in patients that underwent EL fluctuates around 32%.9
Combined drug and endoscopic treatment
Endoscopic sclerotherapy plus drug treatment
NSBBs alone have been compared with NSBBs combined with ES in 3 RCSs with a total of 277 patients and less rebleed risk was shown with the combined treatment, but no differences in the mortality rate were observed.31,55,127,193
On the other hand, 10 RCSs comparing NSBBs with ES, that included 862 patients, showed no difference in terms of rebleeding and mortality (7 vs. 2%), but there were fewer side effects in the NSBB group (2 vs. 22%).55,127
Endoscopic ligature plus drug treatment
Two RCSs demonstrated the superiority of the combination of EL plus NSBB therapy compared with drug therapy or EL alone.190,194 Rebleed frequency was 14% and 23% in the combined treatment group (EL plus nadolol) compared with 38% and 47% in the group with the procedure alone. Therefore the combination of EL with NSBBs is highly recommended in the secondary prophylaxis of esophageal varices.
The combination of NSBBs plus ISMN compared with EL has been evaluated in 3 RCSs and the results were contradictory. One study showed the combined drug therapy to be beneficial,195 another showed EL to have better results,196 and there was no difference between the two treatments in a third study.197 These study results for both treatment strategies were similar in relation to variceal rebleed prevention, ranging between 30% and 35%.
Recommendations:
• EL is superior to ES in secondary prophylaxis of variceal bleeding. (Level of agreement 9).
• ES is not recommended as secondary prophylaxis. (Level of agreement 9).
• NSBB treatment is superior to ES in the secondary prophylaxis of variceal bleeding due to the lower percentage of side effects. (Level of agreement 9).
• The combined treatment of NSBBs plus EL is currently the best option for secondary prophylaxis of variceal bleeding. (Level of agreement 9).
Other therapies
Surgery
Surgery is very effective in rebleed prevention. The most common surgical diversions are the distal splenorenal shunt (Warren) and gastroesophageal devascularization (Sugiura). The main disadvantage in these procedures is the greater frequency of encephalopathy, but with no impact on survival. The patients with compensated cirrhosis, especially Child-Pugh class A patients, are the group that benefits the most from surgical diversion.11,198
Transjugular intrahepatic portosystemic shunt
As a diversion procedure TIPS is effective in preventing rebleed, without being the first-line treatment. It is used when there has been medical or endoscopic treatment failure.199
TIPS has been extensively studied and compared with other treatments11,200,201; a meta-analysis demonstrated its superiority over endoscopic therapy in long-term rebleed prevention (19 vs. 47%), even though this advantage did not result in a better survival rate (27 vs. 27%). TIPS is associated with a significantly higher portosystemic encephalopathy rate (34 vs. 19%). Similar results were obtained when TIPS was compared with drug treatment; TIPS had the significant advantage, achieving a lower rebleed rate.201 Contrastingly, in the drug group, portosystemic encephalopathy is approximately half that of the TIPS group, whereas there was no difference in the survival rate between groups.
TIPS has also been compared with surgery in the secondary prophylaxis setting.202,203 A multicenter RCS recently published by Henderson, that included 140 Child-Pugh class A or B cirrhotic patients, and had a 2 to 8 year follow-up, did not show any difference in the rebleed, encephalopathy, or survival rates at 2 and 5 years; there was a higher rate of thrombosis, stricture, and need for re-operation in the TIPS group (82%), compared with the distal splenorenal shunt group (11%). Therefore, it has been concluded that the treatment option should be based on the local experience of each center, the possibility of having adequate follow-up, and the logistics of immediate inter vention, when necessary.202
Recommendations:
• Surgical diversion and TIPS are the therapeutic alternatives of choice in secondary prophylaxis, when drug and/or endoscopic treatments have failed and depending on the patient's liver reserve. (Level of agreement 9).
• Surgical diversion can be a viable option in stable cirrhotic patients (Child-Pugh class A), but if chosen, must be carried out in centers that have the necessary experience in either of these 2 surgeries. (Level of agreement 9).
• TIPS is used as recue therapy to prevent variceal rebleed. (Level of agreement 9).
Patients with bleeding due to portal hypertensive gastropathy
PHG causes approximately 25% of all bleeding (acute and chronic) in patients presenting with portal hypertension. The most common presentation is chronic bleeding and anemia. Drug therapy is not currently recommended for preventing acute bleeding (primary prophylaxis) in patients with PHG. However, NSBBs have been recommended for preventing chronic bleeding due to PHG.204,205
Recommendation:
• In patients with chronic bleeding due to PHG, NSBBs and iron supplements are recommended as the first option. (Level of agreement 9).
Financial disclosure
The current Mexican Consensus on Portal Hypertension had the support of the Asociación Mexicana de Gastroenterología, Asociación Mexicana de Hepatología, and the Asociación Mexicana de Endoscopía Gastrointestinal, all of which participated in the organization of meetings, the elaboration of the statements, and the voting process.
Conflict of interests
The authors declare that there is no conflict of interest.
Received 20 January 2012;
accepted 21 January 2013
* Corresponding author at:
Av. Madero y Gonzalitos S/N. Edificio Dr. Rodrigo F. Barragán 2.o piso, Monterrey, Nuevo León, Mexico C.P. 64460.
Tel.: +81 83333664; fax: +81 83486068.
E-mail address: fbosques58@hotmail.com (F.J. Bosques-Padilla).