Non-alcoholic fatty liver disease (NAFLD) is currently one of the main causes of chronic liver disease in Western countries, with a 25% prevalence reported in the general population worldwide. Visceral adiposity and liver fat promote a state of systemic inflammation, predisposing individuals with NAFLD to the extrahepatic pathologies of cardiovascular disease (the most common cause of death in patients with NAFLD), diabetes mellitus, chronic kidney disease, hypothyroidism, polycystic ovary syndrome, obstructive sleep apnea, and an increased risk for presenting with gastrointestinal and extraintestinal neoplasias. Different mechanisms between NAFLD and its association with extrahepatic diseases have been reported, and lipotoxicity is the main cause of inflammatory pathway activation that results in extrahepatic tissue damage.
La Enfermedad por Hígado Graso no Alcohólico (EHGNA) es actualmente una de las principales causas de hepatopatía crónica en el mundo occidental. La prevalencia mundial reportada en población general es del 25%. La adiposidad visceral y grasa hepática propician un estado de inflamación sistémica, predisponiendo a los individuos con EHGNA a enfermedades extra-hepáticas como enfermedad cardiovascular (causa más común de muerte en EHGNA), Diabetes Mellitus, enfermedad renal crónica, hipotiroidismo, síndrome de ovario poliquístico, apnea obstructiva del sueño, así como aumento del riesgo de presentar neoplasias gastrointestinales y extraintestinales. Diversos mecanismos se han reportado entre la EHGNA y su asociación con enfermedades extra- hepáticas, siendo la lipotoxicidad la principal causa de activación de vías inflamatorias que ocasionan el daño tisular extra hepático.
Non-alcoholic fatty liver disease (NAFLD) is a clinical disease characterized by the histologic finding of ≥ 5% macrovesicular steatosis of the hepatocytes in individuals with no significant alcohol consumption (≥ 30g/day for men and ≥ 20g/day for women) or other known cause of chronic liver disease.1,2 It has developed into an important cause of chronic liver disease in Western cities and will become the first underlying cause of liver transplantation within the next 10 years.3–5
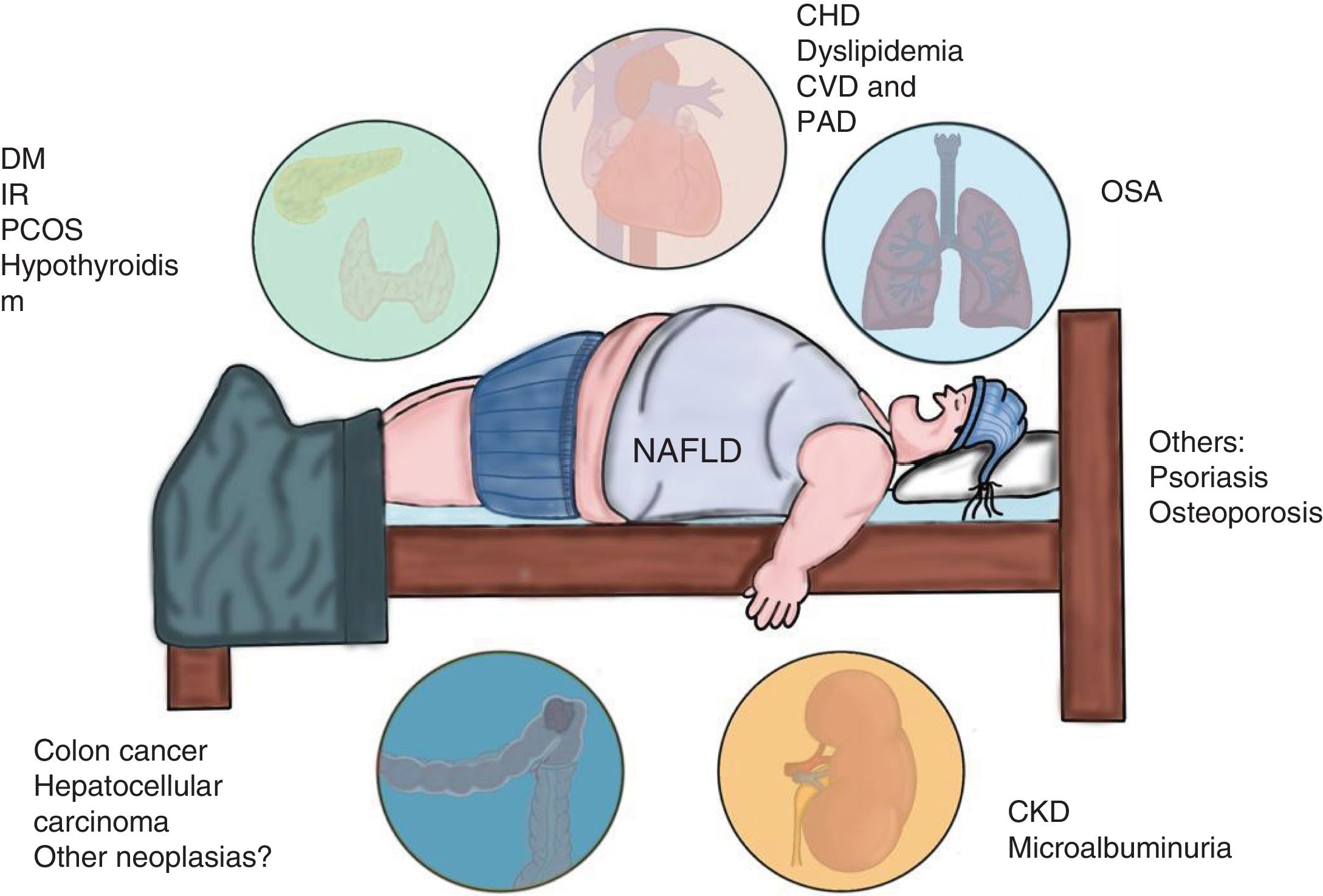
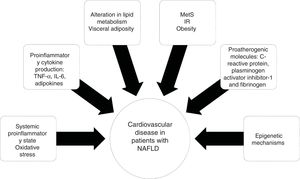
NAFLD is associated with metabolic comorbidities that include diabetes mellitus (DM), insulin resistance (IR), obesity, high blood pressure, and hyperlipidemia, which on their own, increase the risk for cardiovascular disease (CVD).6 In the last 10 years, NAFLD has been identified as a multisystemic disease that affects a variety of organs, and is associated with DM, chronic kidney disease (CKD), CVD, and malignancies, among others (Fig. 1).
Diseases associated with the multi-systemic involvement present in non-alcoholic fatty liver disease (NAFLD).
CHD: coronary heart disease; CKD: chronic kidney disease; CVD: cardiovascular disease; DM: diabetes mellitus; IR: insulin resistance; OSA: obstructive sleep apnea; PAD: peripheral artery disease; PCOS: polycystic ovary syndrome.
The 2015–2016 National Health and Nutrition Examination Survey (NHANES) included more than 11,000 individuals with NAFLD, followed for a mean 14.5 years. The main causes of death were CVD (9.3%) and malignancy (5%), and complications related to liver disease were the cause of death in 0.4%. To date, evidence has indicated that coronary heart disease (CHD) is the main cause of morbidity and mortality in patients with NAFLD, and NAFLD has also been linked to structural and functional myocardial alterations, with or without the coexisting characteristics of metabolic syndrome (MetS).3 A large body of clinical and epidemiologic evidence has suggested that NAFLD not only is associated with liver-related morbidity and mortality, but also may play a part in the development of coronary heart disease, alterations in cardiac structure and function (left ventricular dysfunction, hypertrophy, and heart failure), heart valve disease (aortic valve sclerosis), and arrhythmias (atrial fibrillation).3,7
In a study on approximately 3,000 non-selected patients with DM, the prevalence of CHD, cerebrovascular disease, and peripheral vascular disease was notably higher in patients with NAFLD than in those without the disease, regardless of the risk factors of DM duration, glycemia control, use of lipid-lowering, hypoglycemic, antihypertensive, or antiplatelet drugs, and MetS components. Patients with NAFLD had a markedly higher age and sex-adjusted prevalence (p<0.001) of CHD (10.8% versus 1.1%), cerebrovascular disease (37.3% versus 5.5%), and peripheral vascular disease (24.5% versus 2.5%) than the patients without NAFLD.7
The aims of the present literature review were to recognize the extrahepatic complications associated with NAFLD, giving importance to the cardiovascular impact of that entity, and to review the general pathophysiologic and epidemiologic aspects involved in NAFLD and its complications. Our desire is that the present review contributes to keeping general practitioners and specialists in gastroenterology up to date on the theme, presenting the necessary knowledge for providing opportune and quality care to the patients with that liver disease.
Materials and methodsA search of the literature was carried out utilizing the DynaMed, Google Scholar, and PubMed databases to find systematic reviews, clinical practice guidelines, randomized controlled trials, and epidemiologic studies in English and Spanish, with the terms “steatosis”, “non-alcoholic fatty liver disease”, “steatohepatitis”, “metabolic syndrome”, “mortality”, “neoplasias”, “cancer” “psoriasis”, “hypothyroidism”, “polycystic ovary”, and “cardiovascular risk” and their Spanish equivalents, within the time frame of 2000 and the first trimester of 2019. A manual search of the abstracts was conducted for their inclusion and the references selected were based on their adaptation to the aims of the present review.
EpidemiologyNAFLD is the most common liver disease in Western cities and in relation to different diagnostic methods, age, sex, and ethnicity, it affects 17–46% of adults.8,9 The highest rates have been reported in South America and the Middle East, followed by Asia, the United States, and Europe.10 According to various reports, it presents in 7–9% of persons with normal weight, at an earlier age, with normal liver enzymes, and with greater frequency in women, underlining the fact that thin individuals with NAFLD have a different clinical profile from overweight or obese patients with NAFLD.11–13
Nevertheless, the overall prevalence in adults in the general population has been reported to vary from 3 to 24% and the majority of calculations range from 6 to 14%.14
Screening for NAFLD has been questioned, given the high direct and indirect costs of the tests, the low predictive value of the noninvasive tests, liver biopsy risks, and the lack of treatment effectiveness. However, the aggressive forms, such as non-alcoholic steatohepatitis (NASH), especially when associated with advanced fibrosis, should be identified in at-risk patients (age > 50 years, DM, or MetS) due to its prognostic implications.2
It is not easy to establish the prevalence of NAFLD in the general population because of the need for histologic evaluation. In a study conducted on the general world population, the prevalence of NAFLD was estimated through ultrasound imaging and altered liver function tests in the absence of some other cause of liver disease, resulting in a worldwide prevalence of 25%.8 According to the NHANES III database, the prevalence of NAFLD determined through ultrasound imaging was reported at 19%, and through altered liver enzymes at 24%.9
The majority of patients present with stable disease, but the progression of fibrosis has been shown to occur in patients with NASH, as well as with NAFLD (annual increase in fibrosis of 0.14 and 0.07, respectively). However, a subgroup of patients was identified with rapid fibrosis progression. They presented with a high frequency of mild lobular inflammation or hepatocyte ballooning, compared with non-progressing patients. Even though those subtle differences are insufficient for diagnosing NASH, they could explain the progression.15
Up to April of 2019, the European register of NAFLD had recruited 6,708 patients, 21% of whom had simple steatosis, 5% steatosis and fibrosis, 58% steatohepatitis (with or without fibrosis), and 8% cirrhosis of the liver.16
We know that Mexico is a country whose population incorporates different risk factors for NAFLD. With respect to the epidemiologic data, prevalence in the population has been reported to vary from 14.3 to 50%.17–19
Extrahepatic complications of NAFLDVisceral adiposity and liver fat condition a state of systemic inflammation that appears to predispose individual with NAFLD to extrahepatic diseases.2 The causality of those extrahepatic pathologies is controversial, but their transcendence is unquestionable, given that to date, the morbidity and mortality in those patients is mainly due to CVD, followed by extrahepatic neoplasias.20
Cardiovascular diseaseThe liver plays a crucial role in lipid and glucose homeostasis and therefore is the center of cardiometabolic disease. There is a very complex interaction between the intestine, visceral and subcutaneous adipose tissue, muscle tissue, and the hepatic and cardiovascular systems.21
Numerous studies have been conducted to identify the individuals that may develop CVD, as well as the mechanisms by which liver disease influences that process. Because CVD is the most common cause of death in patients with NAFLD, the ability to identify and modify the cardiovascular risks has become an important target for managing patients with NAFLD.
Several prospective studies have examined the connection between CVD and NAFLD, utilizing a variety of diagnostic techniques, including ultrasound imaging, liver biopsy, and the increase in serum alanine aminotransferase (ALT) and gamma-glutamyl transpeptidase (GGT) levels. The increase in GGT levels is thought to be an indicator of oxidative stress.22 Haring et al.23 found that altered GGT levels were associated with a 2-fold increase in the risk for cardiovascular mortality, in addition to being superior to liver ultrasound in predicting the risk for death.
On the other hand, a prospective study that included patients diagnosed with NAFLD through ultrasound imaging found a greater incidence of serious cardiovascular events during follow-up, regardless of the other known risk factors of smoking, high blood pressure, low density lipoprotein cholesterol levels, MetS, age, and sex.24 A retrospective review with data from the NHANES, showed an increased risk for CVD in patients with NAFLD diagnosed through ultrasound, regardless of liver enzyme levels, but with no increase in mortality.25 In addition to the traditional cardiovascular risk factors, “non-traditional” ones, such as hyperuricemia, hypoadiponectinemia, and vitamin D hypovitaminosis have been observed, along with the relation to CKD, which in turn, increases the cardiovascular risk.20
Subclinical cardiovascular diseaseSubclinical CVD is defined as an imbalance between vasoconstricting and vasodilating substances, especially nitric oxide, which leads to endothelial dysfunction and is considered a state of risk for CVD in asymptomatic patients. Different parameters have been established for detecting early signs of CVD: a) an increase in the thickness of the arterial wall or the presence of carotid plaques identified through ultrasound study, b) CT coronary artery calcium scoring, c) left ventricular dysfunction and cardiac arrhythmias determined through electrocardiogram and echocardiogram, and d) peripheral arterial disease evaluated by the ankle-brachial pressure index.26,27
Subclinical cardiovascular risk evaluationDifferent noninvasive tests have been used in patients with NAFLD to diagnose subclinical CVD. They include carotid intima-media thickness, arterial stiffness, flow-mediated vasodilation, coronary artery calcification (CAC), and epicardial fat.
A systematic review of 7 studies included 1,427 patients with NAFLD and 2,070 controls, showing a significant association between NAFLD and increased carotid intima-media thickness and the presence of carotid plaques.28 The increase in serum ALT and GGT levels was strongly correlated with the degree of thickening of the carotid intima-media, suggesting a relation between the severity of liver disease and the risk for atherosclerotic disease.
In a study on young adults with NAFLD between 20–40 years of age, there was a significant association with subclinical atherosclerosis determined through vascular evaluation that included carotid intima-media thickness, flow-mediated vasodilation, and arterial stiffness.29 CAC is another marker of subclinical CVD that has been linked to the increased risk for cardiac events. The substantial increase in the prevalence of CAC in patients with NAFLD appears to be equal to that in patients with well-established cardiac risk factors, such as smoking and DM.30,31
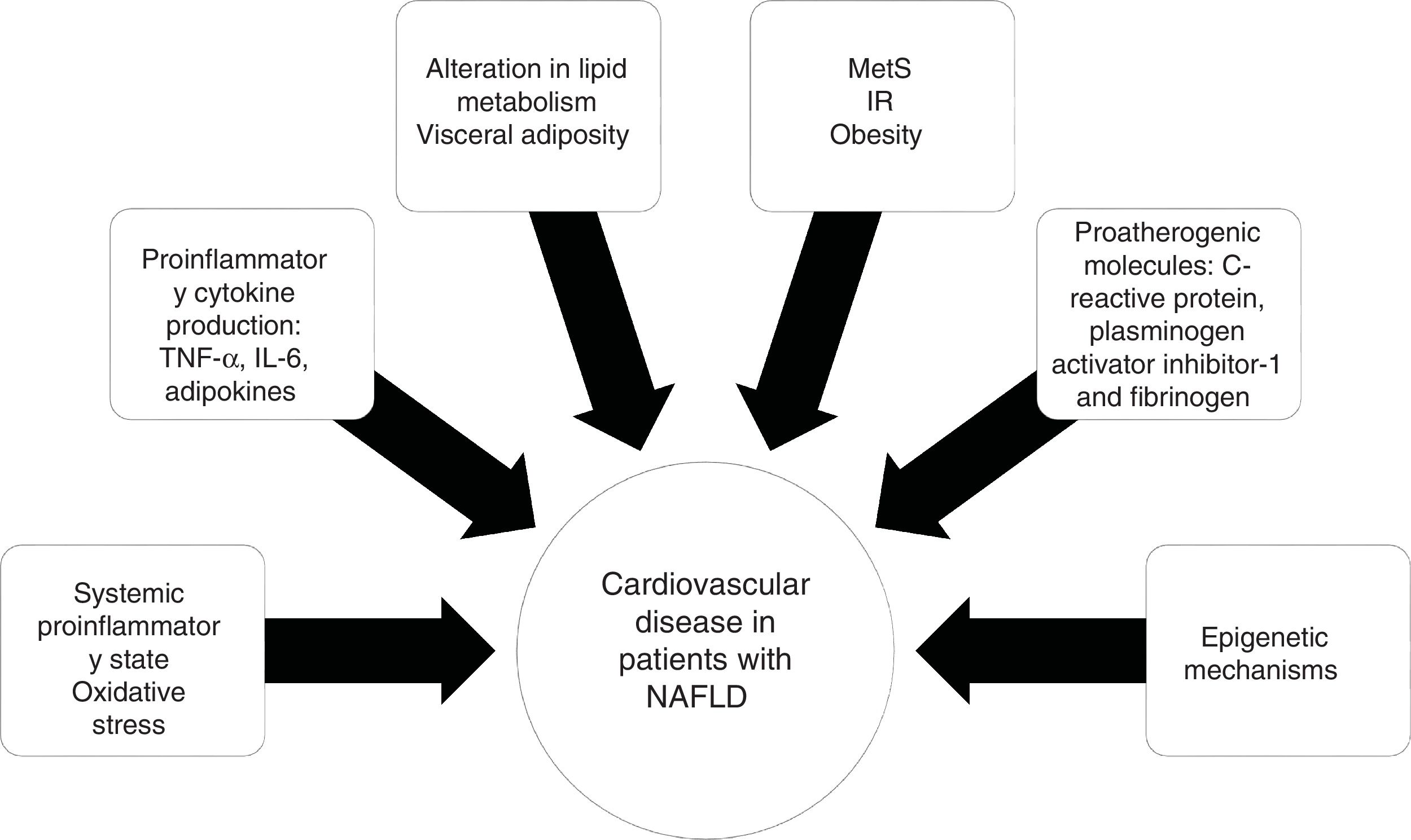
PathophysiologyThe relation between NAFLD and CVD does not seem to be purely related to the overlap of risk factors, but rather to the atherosclerotic effect of hepatic steatosis and steatohepatitis. The supposed mechanisms for accelerating atherosclerotic disease in patients with NAFLD include a proinflammatory state at the systemic level and alterations in lipid metabolism.
IR is a key component in MetS that is strongly linked to the development and progression of NAFLD.32 Obesity, specifically visceral adiposity, causes an increase in the hepatic accumulation of free fatty acids, as well as a decrease in their oxidation and an alteration in glucose metabolism, contributing to hepatic IR.
Visceral obesity is associated with proinflammatory cytokine production, such as tumor necrosis factor-alpha (TNF-α) and interleukin-6 (IL-6), adipokines, and macrophage infiltration, resulting in systemic inflammation and the consequent hepatic production of pro-atherogenic molecules, such as C-reactive protein, plasminogen activator inhibitor-1, and fibrinogen, finally causing endothelial dysfunction and atherosclerosis.33 The role of epigenetic mechanisms, such as DNA methylation, could explain the gene-environment interactions that influence CVD20 (Fig. 2).
As MetS components, DM and IR are strongly related to the prevalence of NAFLD. Thus, NAFLD has been shown to be an independent risk factor for the development of DM. In the study by Framingham, individuals with NAFLD at baseline were confirmed to be more susceptible to developing subsequent DM2 that those that did not have NAFLD.20 Overweight and obesity in individuals with NAFLD that underwent screening for DM with the glucose tolerance test had a greater range of intolerance to carbohydrates, compared with an overweight or obese control group with no NAFLD (75% vs 25%).34
Both liver fat and DM are significantly associated with the presence of IR, suggesting that the increase in liver fat plays a role in the development of DM. Given the significant risk for developing DM in patients with NAFLD, annual DM screening with glycosylated hemoglobin or the oral glucose tolerance test is recommended.35
PathogenesisThere is a mutual association between adipose tissue dysfunction, NAFLD, and the development of glucose tolerance alteration and DM. The association of steatohepatitis and advanced fibrosis with DM appears to be independent of obesity and linked to the presence of IR.32 Lipotoxicity results from the accumulation of toxic metabolites and triglycerides in the muscle, liver, and beta pancreatic cells, with inflammatory pathway activation that results in IR. Excess free fatty acids in NAFLD leads to IR in the skeletal muscle that is proportional to the degree of adipose tissue with IR. Adipose tissue dysfunction results in the development of the metabolic abnormalities of dyslipidemia, muscle and liver IR, hepatic steatosis, and steatohepatitis.36,37
Chronic kidney diseaseCKD is defined as an estimated glomerular filtration rate (eGFR) reduction, or microalbuminuria or proteinuria. CKD often affects persons with the known metabolic risk factors of high blood pressure and DM. New studies have revealed that NAFLD is independently associated with the increased prevalence of CKD and therefore patients with NAFLD appear to have greater ranges of CKD, compared with patients that do not present with NAFLD.38
On the other hand, in a study on 11,469 adults in the United States, no increased risk for CKD was found in patients diagnosed with NAFLD through ultrasound imaging after MetS remission.39 However, recent evidence from a cohort study on adults with no CKD confirmed that NAFLD was associated with a greater risk for developing CKD. It was also reported that NAFLD might negatively affect kidney function and that patients could need to be carefully monitored due to an increased long-term risk for kidney damage.40 Thus, CKD screening through the yearly evaluation of eGFR and microalbuminuria is recommended in patients with NAFLD.
PathogenesisNAFLD is currently suggested to be a marker for CKD. The underlying lipotoxicity, oxidative stress, and chronic inflammation in NAFLD are considered important contributing factors to the pathogenesis of CKD through inflammatory pathway activation, endothelial dysfunction promotion, altered fetuin A and adiponectin levels, and adhesion molecule over-regulation,41 in addition to increased atherogenesis. Unfortunately, kidney biopsy was not performed in the majority of the studies showing said association, and therefore it is not known whether NAFLD is associated with a particular histologic type of CKD.20
Adiponectin and fetuin A are IR mediators and key ligands between obesity, liver disease, and kidney disease. Fetuin A is an IR promotor, secreted by the liver, that regulates adiponectin production by the adipose tissue. Low levels of adiponectin are correlated with microalbuminuria and proteinuria. The mechanism appears to be reduced in the kinase protein activated by AMP 5´ in the podocytes, resulting in albuminuria.42
Extrahepatic neoplasiasObesity increases the risk for mortality in all types of cancer. Even though liver fibrosis and cirrhosis are clearly related to the development of hepatocellular carcinoma, patients with NAFLD appear to have a greater range of extrahepatic neoplasias. Colon cancer in particular is strongly association with NAFLD. In addition, in patients with NAFLD, the presence of other malignant tumors at the gastrointestinal level (esophagus, stomach, pancreas) and the extraintestinal level (kidney, prostate in men, and breast in women) is well known, converting the different types of cancer into one of the three main causes of death in those patients.43
Colorectal cancerColorectal cancer (CRC) is the third most common cancer worldwide, and its modifiable risk factors include obesity, a sedentary lifestyle, and a diet rich in animal fat and low in fiber. Wong et al. found a higher prevalence of colorectal adenomas in individuals with steatohepatitis determined through biopsy (51% vs 25.6%) and neoplasias (34.7% vs 14%), compared with patients with hepatic steatosis with no inflammation. That finding persisted after the correction of other identifiable risk factors.44
Practically all the recent studies have shown an elevated prevalence of colorectal lesions in patients with NAFLD, compared with those that did not have the disease. In their study on a population of 2,917 participants that underwent colonoscopy, abdominal ultrasound, and liver function tests, Hwang et al.45 showed that the prevalence of NAFLD was 41.5% in the patients that presented with adenomatous polyps of the colon, compared with 30.2% in the group that did not present with polyps. In the multivariate analysis, the presence of fatty liver was associated with a 3-fold increased risk for developing adenomatous polyps in the colon. In a later study conducted in Korea on a population of 5,517 women, those that presented with hepatic steatosis had a 2-fold increased risk for developing adenomatous polyps and a 3-fold higher risk for presenting with CRC, compared with the controls.46
One of the most important findings about that relation showed that in patients with NAFLD, those that had histologic evidence of steatohepatitis had a higher risk for developing colon cancer. There are studies showing that CRC is much more frequent in patients with NASH, compared with patients with NAFLD and no inflammation (51.0% vs 25.6%). The evidence of steatohepatitis remained strongly associated with the elevated risk for adenomas (OR 4.89), as well as for advanced neoplasias of the colon (OR 5.34), even after adjusting the demographic and metabolic risk factors.47
The current guidelines for CRC screening do not recommend performing adjustments for patients with NAFLD or steatohepatitis. However, given their clear association, adherence to those guidelines should be strongly recommended.48
Other digestive and extradigestive neoplasiasAs mentioned before, the association, and possible risk, between NAFLD and other malignant tumors has been described. An example is breast cancer, in which a study reported prevalence of up to 63% of patients with fatty liver, compared with 48% in the controls.49 Another clear example is that of malignant esophageal neoplasias, mainly adenocarcinoma, in which obesity has been demonstrated to increase the risk up to 4-fold, compared with the controls, and in which the presence of visceral fat (including fatty liver) is independently associated with the presence of reflux for developing those types of tumors.50 An even greater association between visceral fat and neoplasia was observed in pancreatic cancer. The relative risk (RR) for developing that neoplasia was shown to increase 1.11 (95% CI: 1.05–1.18) for every 10cm of increase in the abdominal perimeter. Metabolic syndrome and the simultaneous presence of fatty liver and fatty pancreas have been identified as neoplastic risk factors, with a RR of 1.58 (p<0.0001) for developing pancreatic cancer, especially in women, possibly mediated by reduced physical activity, high-calorie food consumption, elevated fat consumption, low fiber consumption, and oxidative stress.51
Finally, two more neoplasias found to have a certain association with NAFLD are kidney cancer and prostate tumors. A European study found a linear increase in the risk for developing kidney cancer in patients with components of metabolic syndrome, compared with controls (43% in men and 40% in women), even after making adjustments for the factors of smoking, diet, and heredity.52 In another study conducted on 118 patients that underwent surgical management for renal cell carcinoma, serum adiponectin levels were measured. The authors found that the adiponectin levels were inversely proportionate to the severity of the neoplasia, finding lower levels in patients with metastatic disease.53 There is less solid evidence regarding prostate cancer. In that search, we only found one study that described the association of different neoplasias in 1,600 patients with NAFLD demonstrated through ultrasound study versus 1,600 controls that were carriers of hepatitis C (HCV).54 The authors reported that prostate cancer presented in 12.6% of the patients with fatty liver, compared with 3.5% of the patients with HCV. They also found that the incidence of prostate cancer was higher in patients with NAFLD than in the general population.
EndocrinopathiesNAFLD has been associated with a variety of endocrinopathies, including polycystic ovary syndrome, hypothyroidism, growth hormone deficiency, hypogonadism, hypopituitarism, and hypercortisolemia.
Both the deficiency and excess of androgens play a role in the development of hypogonadism in men and polycystic ovary syndrome in women.34
Polycystic ovary syndromePolycystic ovary syndrome (PCOS) is a reproductive disease characterized by an excess of androgens, associated with obesity and IR. Numerous cohort studies have shown greater prevalence of NAFLD in women with polycystic ovary syndrome.55 There is little current evidence that suggests a direct effect of androgen excess in the pathogenesis of NAFLD but due to the risk factors they share, PCOS should be contemplated and ruled out in women with NAFLD that present with infertility and menstrual alterations.35
The data from several studies reveal that the prevalence of NAFLD in PCOS varies from 35 to 70%, compared with 20 to 30% in women that do not present with PCOS, with similar age, BMI, and hip circumference.56 In their study on 600 white women diagnosed with PCOS, Macut et al.57 reported that NAFLD was more prevalent in PCOS cohorts with increased abdominal circumference, elevated lipid accumulation product, increased IR, and elevated serum levels of total cholesterol and triglycerides. Finally, we can state that what links PCOS pathophysiologically to NAFLD is IR. That is explained by failed lipolysis suppression in the adipose tissue, resulting in an increase in the entrance of free fatty acids into the liver. Visceral adiposity and hypertriglyceridemia play an important role in the pathogenesis of NAFLD in women with PCOS, as has been shown by the decrease in hepatic steatosis after weight loss and reduced serum triglycerides with the use of omega-3 fatty acids.58
HypothyroidismThyroid dysfunction, previously related to obesity and MetS, appears to also have a strong association with the development of NAFLD.59 Hypothyroidism is a metabolic disease characterized by a low level of thyroid hormone (TH) and elevated serum thyroid-stimulating hormone (TSH), which is strongly associated with NAFLD. A 15.2–36.3% prevalence of hypothyroidism in patients with NAFLD has been reported, with a national prevalence of 3.7%.60 A correlation between the dose-dependent level of TSH and the risk for NAFLD has been found in several studies. A solid association has also been reported between subclinical hypothyroidism and low-normal thyroid function with the risk for NASH and advanced fibrosis.61 With respect to the pathophysiologic mechanisms implied, the relation between thyroid autoimmunity and cardiovascular risk has been demonstrated. The authors of that study found that thyroid autoimmunity was positively associated with HbA1c, HOMA-IR, obesity, central obesity, hyperlipidemia, and MetS, especially in women (25% MetS).62
Ferrandino et al.63 conducted a study on mice and demonstrated that the pathogenesis of NAFLD induced by hypothyroidism occurs due to intrahepatic and extrahepatic mechanisms. Hypothyroidism induces NAFLD through a pleiotropic effect of the THs on insulin secretion and the adrenergic stimulation of lipolysis in adipose tissue. The decrease in serum TH levels alters insulin secretion, leading to altered lysis suppression and an increase in the transport of fatty acids to the liver, accumulated as triglycerides, where they induce NAFLD. Lipid accumulation in the liver induces IR, resulting in deficient suppression in the production of post-prandial endogenous glucose, which is why, based on those data, annual TSH screening should be considered in patients with NAFLD.64,65
Obstructive sleep apneaObstructive sleep apnea (OSA) is a medical condition that affects approximately 4% of the general population, with a greater prevalence in persons with obesity (25–35% incidence), and is strongly associated with NAFLD,66 in addition to being an important risk factor for steatohepatitis and advanced fibrosis. The chronic and intermittent hypoxia that occurs in OSA conditions an increase in the production of proinflammatory cytokines, oxidative stress, and IR, directly affecting fibrogenesis through factors that induce hypoxia. There are no current prospective data on the incidence of OAS in patients with NAFLD or that indicate that the appropriate management of OAS could affect the progression of NAFLD.67,68
PsoriasisPsoriasis is not an isolated pathology of the skin, but rather a systemic condition involving multiple organs and systems. Due to the high frequency of MetS in psoriasis, the high incidence of NAFLD in that group of patients is not surprising. The first study by Roberts et al.69 to confirm the increased risk for NAFLD in patients with psoriasis showed a general prevalence of NAFLD of 47%. The prevalence of NASH confirmed by biopsy was 22% and one-third of those patients had advanced fibrosis.69 The presence of NAFLD has been related to greater psoriasis severity and an increased risk for articular damage. In turn, individuals with concomitant NAFLD and psoriasis are more prone to develop liver fibrosis, compared with non-psoriatic controls.70 The etiopathogenic link between both is the state of chronic inflammation and IR. Some proinflammatory cytokines synthetized by lymphocytes and keratinocytes in psoriatic skin, including IL-6, IL-7, and TNF-α, can contribute to IR, a common characteristic with NAFLD. Obesity is one of the main comorbidities that links NAFLD and psoriasis. The epidemiologic relation between obesity and psoriasis, especially in the severe forms, acts bidirectionally, so that obesity favors psoriasis and its severity, and vice versa.71
ConclusionsCurrent knowledge holds that NAFLD should be considered a multisystemic disease that identifies a subgroup of the population with an increased risk for developing serious chronic complications. The clinical impact of NAFLD goes beyond the mortality linked to liver disease: it is associated with cardiovascular morbidity and mortality, CKD, OAS, osteoporosis, psoriasis, CRC, iron overload, and several endocrinopathies. CVD and its associated mortality have acquired a special emphasis, given that in the treatment of all disease, goals must be set. One of the highest priority objectives in NAFLD should be to prevent cardiovascular morbidity and mortality. Inflammation, fibrosis, and neoplasias should also be simultaneously included, but at present there are no clear objectives related to their prevention. Given the lack of current treatments focused on preventing the progression of liver disease, the model for first-line therapy should be the prevention of cardiovascular complications and the detection and treatment of extrahepatic complications of NAFLD.
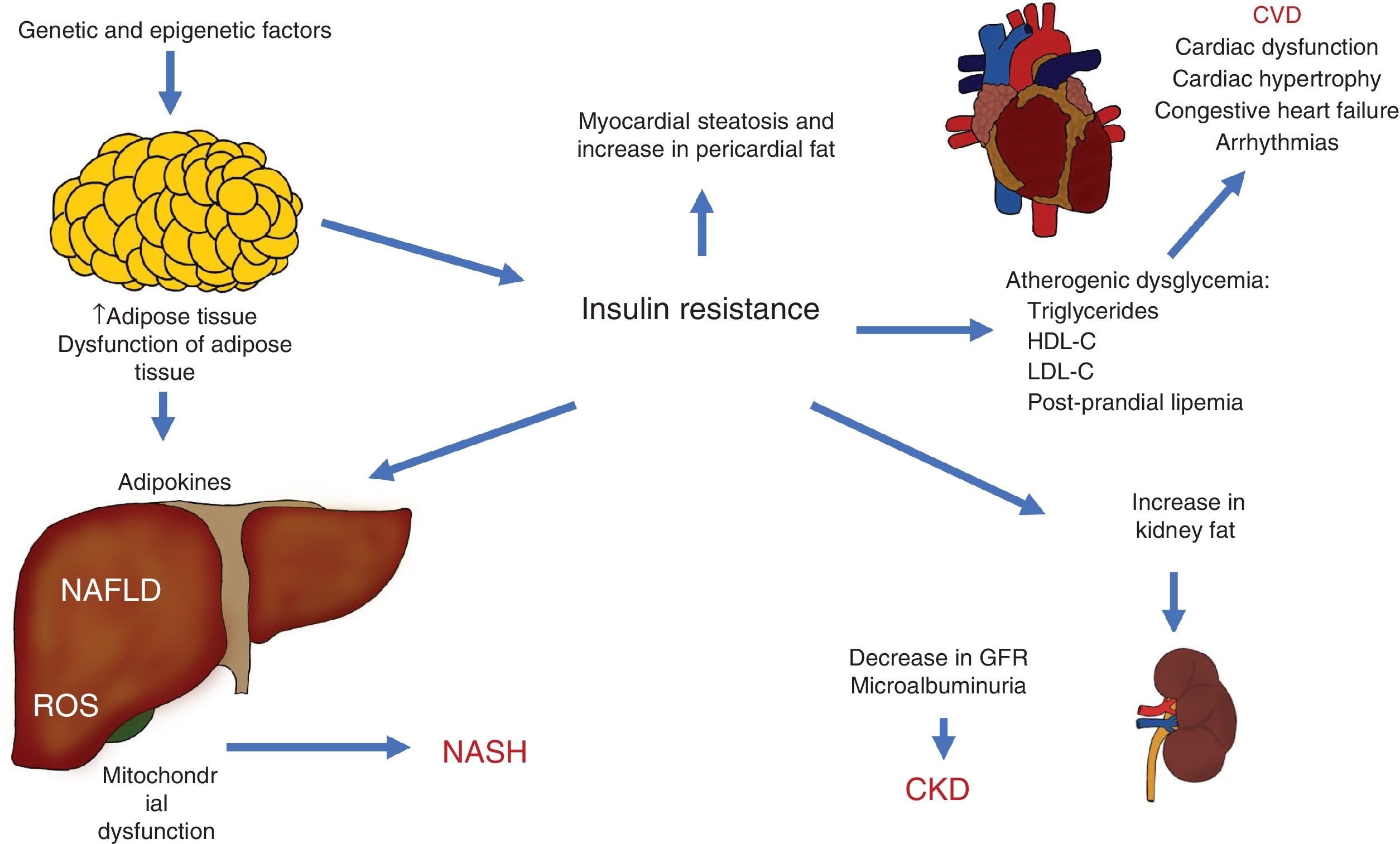
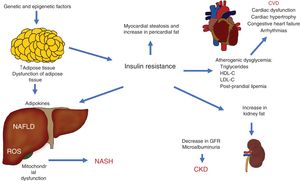
The different pathophysiologic mechanisms that contribute to NAFLD interact with each other, signifying that one individual can present with different complex clinical situations that can be precisely explained. The expansion of adipose tissue, together with IR, are basic mechanisms that connect CVD, CKD, and other complications (Fig. 3).
Pathophysiologic interactions that link cardiovascular disease (CVD), chronic kidney disease (CKD), and other complications observed in non-alcoholic fatty liver disease (NAFLD).
GFR: glomerular filtration rate; HDL-C: high density lipoprotein cholesterol; LDL-C: low density lipoprotein cholesterol; NASH: non-alcoholic steatohepatitis; ROS: reactive oxygen species.
Current medical evidence recommends screening for the highly prevalent conditions of DM, CVD, kidney disease, hypothyroidism, polycystic ovary syndrome, and obstructive sleep apnea, leading the clinician to emphasize lifestyle changes with respect to exercise, weight loss, and smoking, as well as drug treatments that can modify the extrahepatic complications associated with NAFLD. Finally, the current clinical evidence associating NAFLD with several extrahepatic complications mainly comes from observational studies, with short follow-up periods. Therefore, longitudinal studies are required that can determine the baseline and follow-up cardiometabolic risk profiles, to thoroughly evaluate the real impact of NAFLD on the microvascular and macrovascular damage of those systemic diseases.
Ethical disclosuresThe present work did not require patient informed consent because it is a review article.
The present work did not require the authorization of ethics committees because it is a bibliographic review.
The authors declare that no personal data enabling patient identification appear in this article.
Financial disclosureNo financial support was received in relation to this article.
Conflict of interestThe authors declare that there is no conflict of interest.
Please cite this article as: Velarde-Ruiz Velasco JA, García-Jiménez ES, García-Zermeño KR, et al. Complicaciones extrahepáticas de la enfermedad por hígado graso no alcohólico: impacto más allá del hígado. Revista de Gastroenterología de México. 2019;84:472–481.