Exercise in cirrhosis of the liver is an emerging topic in hepatology. Despite the known benefits of exercise in the general population, there are currently few studies addressing that issue in relation to cirrhosis and more evidence is still needed. Even though some studies have reported an acute, exercise-induced increase in the hepatic venous pressure gradient (HVPG), the opposite (a decrease in the HVPG) has been shown by recent data after an exercise program carried out for>14 weeks. In addition to that benefit, improvement has been described in the metabolic profile, quality of life, muscle mass, cardiopulmonary function, and nutritional status. Together, those features make exercise in cirrhosis a very attractive intervention. However, certain aspects must be taken into account before prescribing exercise in that population and they include cardiovascular risk, musculoskeletal disorders, and complications related to cirrhosis. After considering those factors, an individually tailored exercise program should be developed for each patient, according to the points stated above and the desired goal. Information about exercise-limiting factors, type of exercise prescribed, monitoring methods, and concomitant nutritional therapy is provided in the present review.
El ejercicio en la cirrosis hepática es un tópico emergente en hepatología. A pesar de los beneficios conocidos del ejercicio en la población general, actualmente hay pocos estudios que aborden este tema en la cirrosis y aún se necesita más evidencia. Aunque algunos estudios han mencionado un aumento agudo en el gradiente de presión venosa hepática (HVPG) inducido por el ejercicio, existen datos actuales que muestran lo contrario (una disminución en HVPG) después de un programa de ejercicio durante>14 semanas. Además de este beneficio, se han descrito mejorías en el perfil metabólico, la calidad de vida, la masa muscular, la función cardiopulmonar y el estado nutricional. En conjunto, esto hace que el ejercicio en la cirrosis sea una intervención muy atractiva. Sin embargo, ciertas consideraciones deben tenerse en cuenta antes de prescribir ejercicio en esta población, incluido el riesgo cardiovascular, la enfermedad osteomuscular y las complicaciones relacionadas con la cirrosis. Después de considerar estos factores, se debe estructurar un programa de ejercicio individualizado en cada paciente, de acuerdo con lo anterior y el objetivo que se busque. En esta revisión se presenta la información sobre las limitaciones, la prescripción del tipo de ejercicio y los métodos utilizados para su monitorización, así como el tratamiento nutricional concomitante.
For nearly 20 years, there has not been sufficient information in the medical literature for supporting and recommending physical exercise as a useful intervention in patients with cirrhosis of the liver and portal hypertension. The evidence for not recommending exercise in cirrhosis was based on findings from 2 studies with small samples. In the first, an acute increase in the hepatic venous pressure gradient (HVPG) was found in patients while they exercised on a cycle ergometer.1 The second study produced the same finding of increased HVPG, which was prevented in the group that received a beta-blocker before exercising. Given that evidence, exercise was not considered recommendable in patients with cirrhosis due to the potential risk for inducing variceal bleeding.2
One of the most important observations in those 2 studies was that the increase in the HVPG was acute, occurring while the patient was exercising on the cycle ergometer. That response was similar to the normal physiologic increase in systemic blood pressure observed in healthy patients while they exercised.3,4 It could thus be supposed that the increase in portal pressure during exercise was a normal physiologic response that could be modified under specific circumstances, such as a physical training program. The adaptation by the body, and specifically certain organs, to exercise has been documented in cardiopulmonary diseases, such as heart failure, chronic obstructive pulmonary disease, and post-acute myocardial infarction.5,6
The initial evidence showing that physical exercise was safe for cirrhotic patients with portal hypertension came from a small randomized controlled study in which the effect of supervised aerobic exercise was evaluated. It found that chronic exercise did not increase the HVPG, but actually lowered it.7 Similar results were reported in a subsequent non-controlled study in patients with cirrhosis due to NAFLD.8 Exercise has numerous potential benefits beyond its safety in terms of portal pressure (and even better, the decrease in HVPG), making it a very attractive intervention considered first-line treatment in patients with cirrhosis and portal hypertension. Those benefits include improvement in cardiopulmonary and metabolic function, in body composition, and in quality of life.7,9,10
Despite the abovementioned benefits of exercise, at present there are no guidelines or recommendations on exercise prescription in cirrhosis, the limiting factors that should be considered before initiating an exercise program, intervention follow-up, and the importance of concomitant nutritional therapy. The aim of the present review is to provide information that serves as a guide for the care of patients with cirrhosis that are undergoing an exercise program.
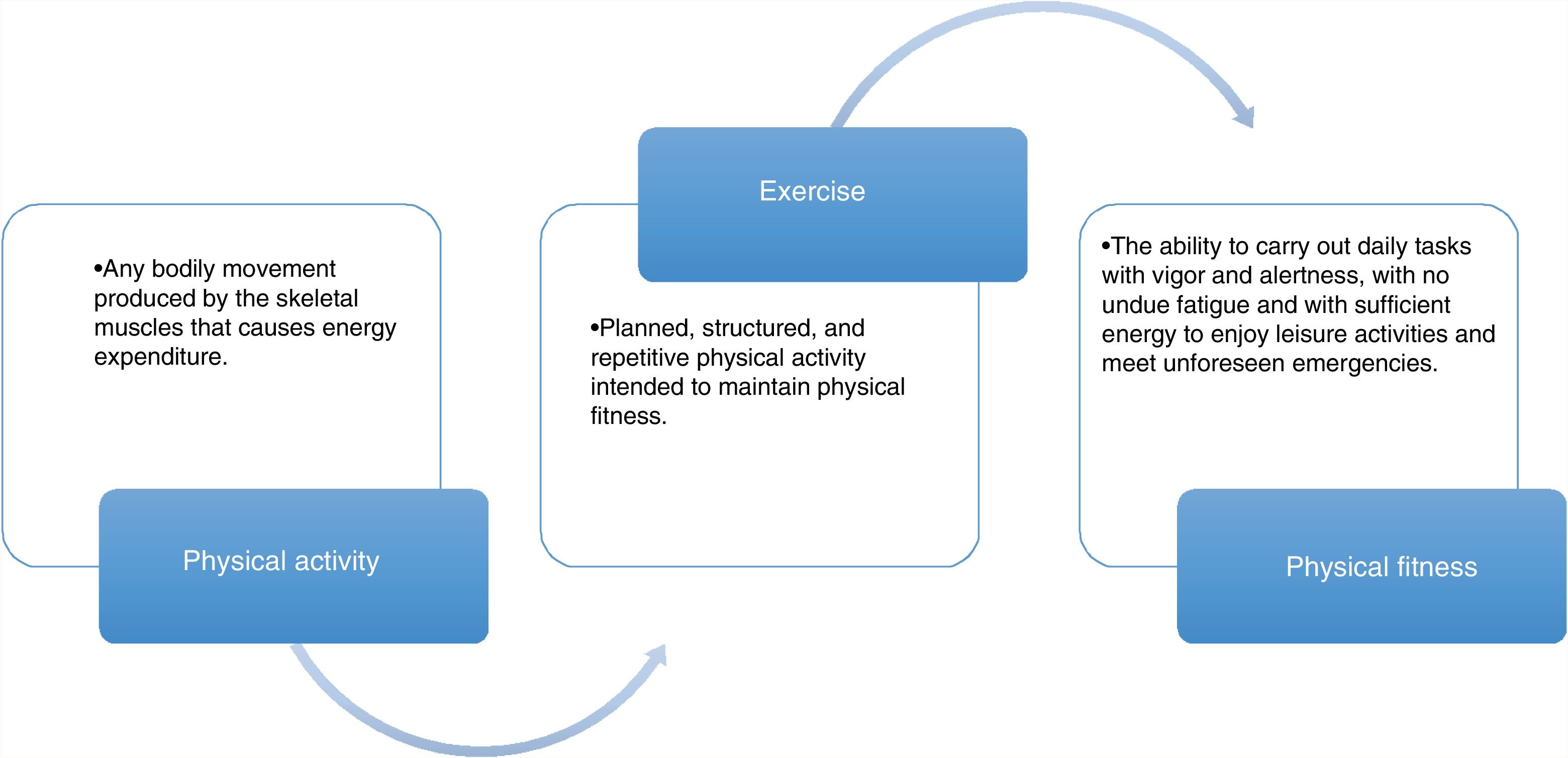
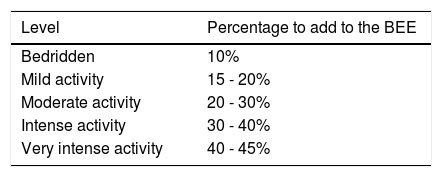
Association between physical activity, exercise, and physical aptitudeTo understand the importance of exercise in cirrhosis, some basic definitions need to be understood, before the evaluation and prescription of an intervention in patients. Even though definitions vary, depending on the organization they come from (fig. 1), and are derived from non-cirrhotic populations,11 the final aim of any exercise program should focus on 3 main points: a) to maintain functionality while the patient is receiving drug treatment or is waiting for definitive treatment (OLT), b) to achieve and maintain the independence of the patients, and c) to improve quality of life. When the body of the patient is “functional”, it allows him/her to maintain a minimum level of functionality, enabling the use of the different bodily systems (central nervous system, muscle system, cardiopulmonary system) to carry out the basic necessities in daily life, when not completely bedridden.
A functional patient is independent and satisfies the basic needs of daily life, including cooking, bathing alone, walking unassisted, etc. Finally, the integration of functionality and independence enables the patient to interact with others and limits comorbidities and costs related to the disease, signifying a better quality of life (fig. 2).
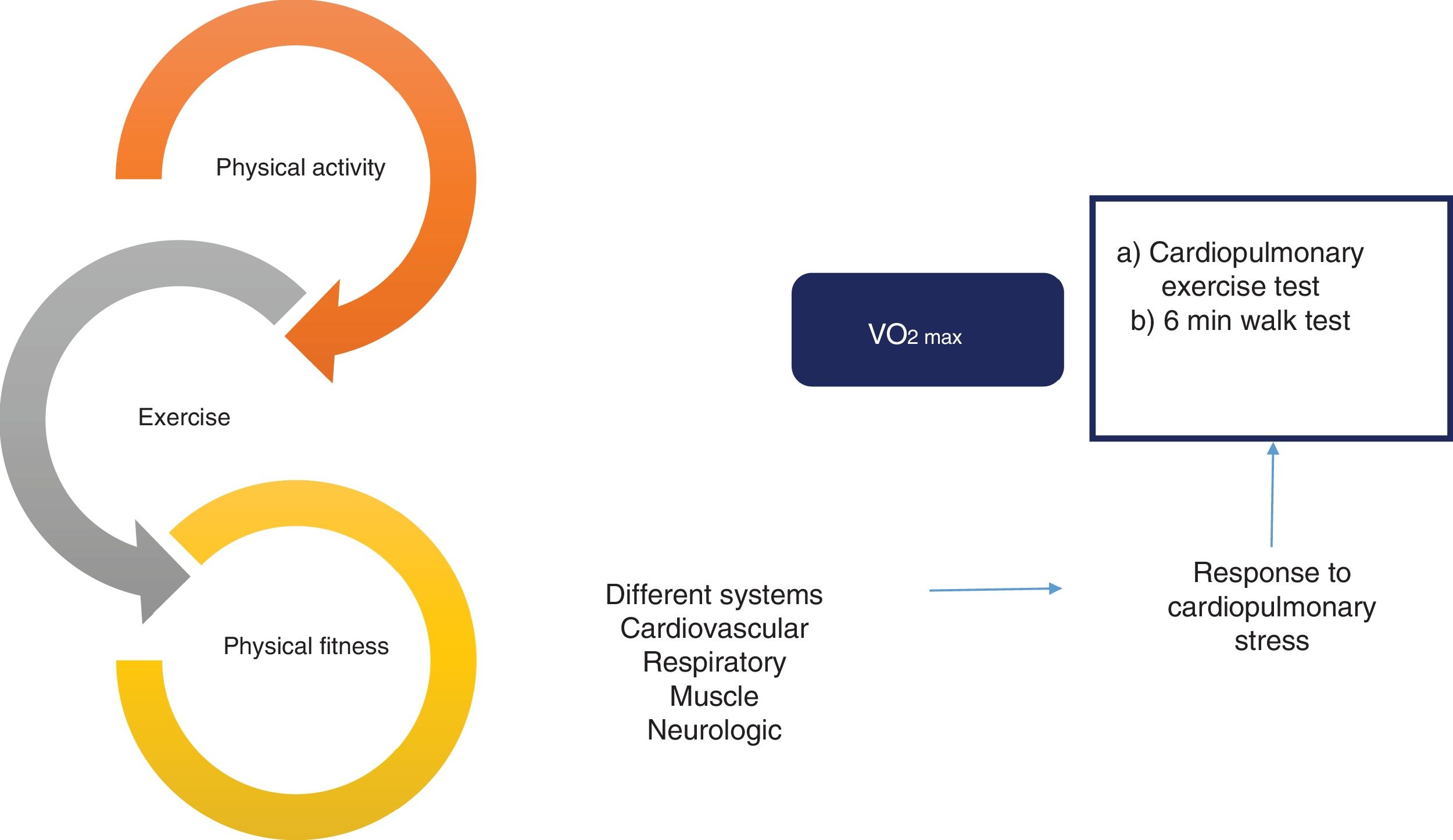
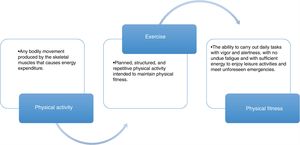
Effect and evaluation of the interrelation between physical activity, exercise, and physical fitness. There is a close relation between physical activity, exercise, and physical fitness that constitutes a continuum with an impact on multiple systems of the organism, with the cardiovascular, respiratory, muscle, and neurologic systems among the most important. That effect is usually evaluated through the response to cardiopulmonary stress, utilizing different methods, such as the cardiopulmonary exercise test and the 6min walk test. Finally, maximum oxygen intake (VO2max) is the most widely studied objective parameter in relation to physical fitness.
The integration of all those concepts aids in becoming aware of the importance of physical activity in the patients with cirrhosis, in the planning and tailoring of the exercise program, and finally, in visualizing the ultimate goal for the patients, centering the program on that aim.
Monitoring of physical activity in patients with cirrhosisWhen a patient with cirrhosis is evaluated for the purpose of undergoing an exercise program, one of the basic questions is how to follow the changes in the variables that indicate physical aptitude, considering the resources available in the center in which the patient is evaluated, and to know the limits and benefits of each method. Follow-up is an obligatory step after beginning any intervention related to exercise in patients with cirrhosis, and 2 objectives must be considered: 1) monitoring the patients while they are exercising, and 2) evaluating the final effect of the exercise on physical aptitude and physical fitness.
Physical activity can be monitored in patients with cirrhosis both in the hospital or in an outpatient setting. The majority of information on that population comes from supervised programs (in-hospital), in which there was direct supervision by a trained team (nurses, physicians, physical therapists). Some of those approaches include the monitoring of heart rate (HR) during exercise, with the goal of reaching 60-70% of the maximum HR (HRmax=220−age, among other formulas that could be more exact, such as HRmax=207−[0.7 x age]).12,13 Other methods for monitoring physical activity in those patients are direct supervision by a specialist in physical activity/rehabilitation that guides the exercise, but without an objective method to measure aspects such as HR, metabolic equivalents (METs), etc.
Even though there are recent guidelines on exercise prescription in healthy adults, as well as on those with different diseases (the American College of Sports Medicine [ACSM]), they are not designed for the care of patients with cirrhosis. There are still no studies on the cirrhotic population that evaluate the general prescription model of the ACSM, which shall be described further ahead.
However, the most important and difficult challenge is the outpatient monitoring of exercise in patients with cirrhosis. There are methods, such as a pedometer, that are simple, accurate, and fairly inexpensive for achieving that. The most practical manner to measure the daily physical activity in cirrhotics is counting the number of steps with a wrist accelerometer, which estimates both the number of steps (like a pedometer) and the physical activity in METs. Habitually, the mean number of daily steps are monitored for a period of 2-3 weeks,14 but reliable information is obtained through a 3-day register (2 days during the week and 1 day at the end of the week).15 Tudor-Locke et al. proposed the following classification of physical activity evaluated by pedometer in non-cirrhotic adults:16a) sedentary <5,000 steps/day; b) low activity 5,000-7,499 steps/day; c) moderate activity 7,500-9,999 steps/day; and d) active ≥ 10,000 steps/day. Those values contrast considerably with that reported in patients on the waiting list for ORT (3,164±2,842 steps/day vs 7,000–14,000 in healthy, non-cirrhotic adults), in which a negative correlation between the severity of the disease and the number of daily steps can be seen.17 Nevertheless, there is currently no consensus that defines the degree of activity according to the number of steps in the population with cirrhosis, making the abovementioned classifications inapplicable to those patients.
The Karnofsky index and Rosow-Breslau questionnaire are among the different subjective scales for evaluating the capacity of patients to perform daily life activities. It is important to mention that the results of those scales are not associated with real physical performance, when compared with other more reliable tools, such as step measurement with a pedometer, but they can be useful when no other instruments are available. The most important limitation of subjective scales is probably that patients perceive they are more active than they really are. Therefore, objective monitoring of physical activity with instruments that can measure the number of steps is of special interest in cirrhosis.18
It is important to know how many steps a patient takes daily and thus design clinical interventions that increase their number. In a previous study, an intervention utilizing a branched-chain amino acid (BCAA) supplement, plus an average of 2,000 steps daily, was evaluated. After 3 months, leg strength measured by ergometer and grip strength improved significantly.19 In patients with heart disease, the pedometer has been reported to serve as a self-motivation tool because of the information it provides, leading to greater competence for achieving the daily step goal.20
The 6-minute walk test (6 MWT) is a commonly used method for evaluating cardiopulmonary fitness in patients with pulmonary, neuromuscular, and neurologic diseases. It is reported to be useful for patients with cirrhosis, as well as in hospitalized patients with hepatocellular carcinoma. The parameter of cardiopulmonary fitness is generally used in clinical trials to quantify the changes resulting from an exercise intervention, to improve aerobic capacity.21,22 The 6 MWT is a practical test that measures the distance a patient can quickly walk on a flat surface in 6min. It evaluates the responses of all the systems involved during exercise, including the pulmonary and cardiovascular system, systemic and peripheral circulation, and muscle metabolism.23 The guidelines established by the American Thoracic Society for performing the 6 MWT recommend using a 30 m long corridor with a flat surface, but not all centers have such long hallways, hindering its application. Therefore, the use of 20 m long corridors has been validated for the performance of said test in patients with cirrhosis.24
In addition to being easy to use, the 6 MWT is inexpensive. It is utilized to evaluate therapies and rehabilitation programs, and as a mortality predictor in candidates for liver transplantation. In fact, patients that walk less than 250 m have a higher mortality rate than those that walk more than 350 m. The respiratory symptoms associated with the test are also correlated with reduced lung capacity, which is a predictive strategy for hospital stay duration and mechanical ventilation after a transplant.25,26 Besides the prognostic importance of the 6 MWT, it is useful for monitoring the effect of different exercise programs in patients with cirrhosis.27,28
Another useful method for evaluating cardiopulmonary fitness is the cardiopulmonary exercise test. It is generally performed on a motorized treadmill or on a cycle ergometer. Cycle ergometry is the most widely used, because it is appropriate for patients with gait instability, orthopedic limitations, or severe obesity.29 Respiratory gas exchange is measured through oxygen consumption (VO2) and carbon dioxide elimination (VCO2), through which maximal oxygen consumption (VO2max) can be calculated. VO2max is the most widely used parameter for evaluating cardiopulmonary fitness and indicates when the actual physiologic limit is reached. That differs from peak oxygen consumption (VO2peak), which is observed when the maximal performance is limited by local muscle factors, and it is used in persons with chronic diseases. Thus, VO2peak would be recommended in patients with cirrhosis.22
To evaluate cardiopulmonary capacity in patients with cirrhosis, the majority of whom perform very little physical activity, it is important to select an appropriate test protocol. Those that maintain the work rate, or gradually and lightly increase it, are recommended. Ideally, the selected protocol should allow the patient to reach an 8-12min period of fatigue-limited exercise. It should be selected according to the comorbidities of the patient and the complications of the cirrhosis.29 On the other hand, if a concomitant evaluation of heart disease (which can be important in patients with NAFLD) is necessary, patients should reach maximum exertion during the test. Nevertheless, there are currently no studies that evaluate which is the best protocol for patients with cirrhosis.13
Exercise-limiting factors and patient evaluation before beginning the exercise programBefore assigning a patient to a physical exercise program, a thorough medical examination of his/her general state of health must be performed that includes evaluating cardiopulmonary function, concomitant diseases, and musculoskeletal limitations. The primary aim of the evaluation in the non-cirrhotic population is to identify any datum (medical condition, sign or symptom) that constitutes a potential risk in relation to the safe participation in an exercise program.13
With that in mind, in the first clinical evaluation for exercise prescription in patients with cirrhosis, the treating physician should focus on 2 areas: 1) the examination of any condition that could keep the patient from safely doing exercise, taking into account the guidelines recommended for the general population (i.e., cardiovascular disease, musculoskeletal disorders, metabolic disease, and kidney disease), and 2) specific complications of cirrhosis.
Regarding exercise prescription, the ACSM guidelines recommend a medical evaluation in patients presenting with cardiovascular, kidney, or metabolic diseases or their signs and symptoms. Even though there are no specific recommendations for patients with cirrhosis, it is logical to suggest that all patients should be evaluated by an experienced physician (with respect to clinical judgement) to identify risk conditions such as those described above, before they begin exercising. Considering that the majority of patients with cirrhosis do not regularly perform physical activity (i.e., 30min of moderate physical activity, 3 times/week over the past 3 months) and the possible coexistence of cardiovascular, metabolic (e.g., in NAFLD), or kidney (kidney dysfunction due to diuretics, hepatorenal syndrome) involvement, all patients with cirrhosis should undergo a thorough medical evaluation.13,30
To achieve an adequate risk evaluation for patients to be assigned to an exercise program, in addition to a complete medical examination, laboratory tests and imaging studies that could help better characterize their general state of health should be considered. Among those tests and studies are lipid profile, fasting glucose, glycosylated hemoglobin, C-reactive protein, electrocardiogram, echocardiogram, spirometry, blood cytology, and chest x-ray. There are specific guidelines that give a detailed description of the usefulness of each of those exams, but in general it can be stated that a thorough clinical evaluation and common sense will dictate which studies should be carried out, including the specialized studies for evaluating cardiac reserve and coronary artery permeability, if they are indicated by risk factors (electrocardiogram, catheterization, thallium-201, or technetium 99m-sestamibi, etc.).31
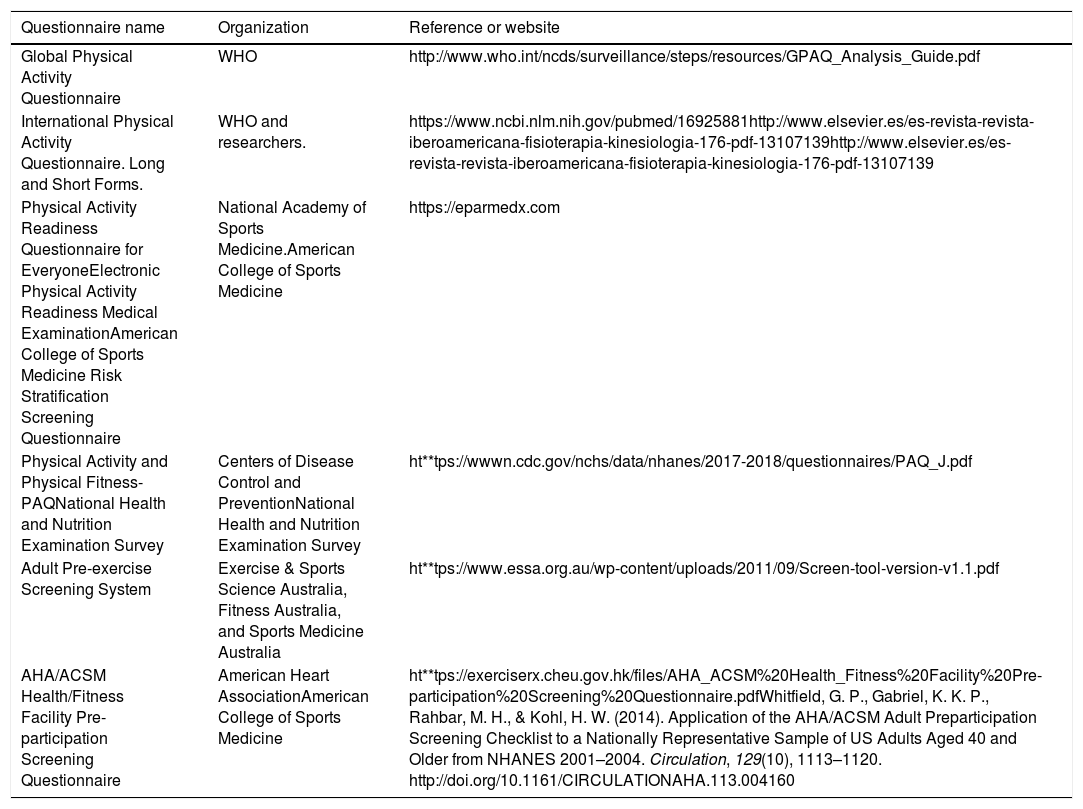
Validated questionnaires are a good option among the different tools for the initial approach to the state of health and physical activity in patients whose goal is to begin an exercise program. Those questionnaires have been developed by different international organizations, including the Canadian Society of Exercise Physiology, the World Health Organization, the ACSM, and the American Heart Association (Table 1). The use of the PAR-Q+questionnaire is advisable, given that it has been widely validated and is recommended in the latest ACSM Guidelines for Exercise Testing and Prescription (ACSM 10).
Questionnaires for evaluating the health of the population before prescribing exercise.
| Questionnaire name | Organization | Reference or website |
|---|---|---|
| Global Physical Activity Questionnaire | WHO | http://www.who.int/ncds/surveillance/steps/resources/GPAQ_Analysis_Guide.pdf |
| International Physical Activity Questionnaire. Long and Short Forms. | WHO and researchers. | https://www.ncbi.nlm.nih.gov/pubmed/16925881http://www.elsevier.es/es-revista-revista-iberoamericana-fisioterapia-kinesiologia-176-pdf-13107139http://www.elsevier.es/es-revista-revista-iberoamericana-fisioterapia-kinesiologia-176-pdf-13107139 |
| Physical Activity Readiness Questionnaire for EveryoneElectronic Physical Activity Readiness Medical ExaminationAmerican College of Sports Medicine Risk Stratification Screening Questionnaire | National Academy of Sports Medicine.American College of Sports Medicine | https://eparmedx.com |
| Physical Activity and Physical Fitness-PAQNational Health and Nutrition Examination Survey | Centers of Disease Control and PreventionNational Health and Nutrition Examination Survey | ht**tps://wwwn.cdc.gov/nchs/data/nhanes/2017-2018/questionnaires/PAQ_J.pdf |
| Adult Pre-exercise Screening System | Exercise & Sports Science Australia, Fitness Australia, and Sports Medicine Australia | ht**tps://www.essa.org.au/wp-content/uploads/2011/09/Screen-tool-version-v1.1.pdf |
| AHA/ACSM Health/Fitness Facility Pre-participation Screening Questionnaire | American Heart AssociationAmerican College of Sports Medicine | ht**tps://exerciserx.cheu.gov.hk/files/AHA_ACSM%20Health_Fitness%20Facility%20Pre-participation%20Screening%20Questionnaire.pdfWhitfield, G. P., Gabriel, K. K. P., Rahbar, M. H., & Kohl, H. W. (2014). Application of the AHA/ACSM Adult Preparticipation Screening Checklist to a Nationally Representative Sample of US Adults Aged 40 and Older from NHANES 2001–2004. Circulation, 129(10), 1113–1120. http://doi.org/10.1161/CIRCULATIONAHA.113.004160 |
In general, cirrhotic patients are less active than non-cirrhotics, and the majority of clinical trials on exercise in cirrhosis have been supervised.7,28,32 There are specific complications of cirrhosis that limit the performance of exercise, such as cardiopulmonary compromise that includes hepatopulmonary syndrome and portopulmonary hypertension, and even pleural effusion (hepatic hydrothorax) in advanced stages of the disease. Those complications alter the normal exchange of gases and the mechanics of the air-fluid interphase, causing abnormal oxygenation (hypoxemia) and hypercapnia, as well as pulmonary restriction (secondary to poor O2 absorption). Other factors that can limit exercise in cirrhosis are peripheral edema, muscle cramps, and ascites, given that they impede the normal walking process and cause pulmonary restriction.33,34
The increase in the HVPG induced during exercise was a constant preoccupation among hepatologists, limiting its prescription, and even more, promoting a sedentary lifestyle in the population with cirrhosis. That recommendation derived from 2 studies that showed an acute increase in portal pressure when the patient exercised on a cycle ergometer. Due to that, the majority of studies are presently conducted on patients at low risk for variceal bleeding. However, current evidence from our group shows that in the long term (i.e., an effect that is not acute), exercise is actually associated with a decrease in the HVPG.7 That finding was later confirmed in a non-controlled study that showed portal pressure improvement after an exercise program in obese cirrhotic patients.8
Even though some studies have demonstrated a negative correlation between the Child-Pugh classification and exercise capacity measured by VO2peak,35 disease severity (evaluated through the Child-Pugh grade and MELD score) is not a contraindication for doing exercise in cirrhosis.36 In the first phases of the disease (i.e., Child-Pugh A), exercise tolerance, measured by VO2peak, is relatively conserved, compared with the final disease stages. Therefore, exercise is not contraindicated based merely on disease severity scores, albeit the focus must be prudent at advanced disease stages and different modifications must be made to the exercise program before assigning the patient to the intervention. That is something that must be considered by the physician in charge of patients with cirrhosis so that immobility is not perpetuated, followed by the consequent decrease in physical capacity.
Exercise prescription in cirrhosisEvidence on the use of exercise in cirrhosisAt present, there is no information available in the literature with respect to exercise prescription or any guidelines on the theme in cirrhosis or portal hypertension. However, data from different clinical trials on the topic, as well as recent guidelines for exercise prescription in the non-cirrhotic population may aid in “guiding” exercise prescription in that clinical setting.The main characteristics regarding the type, duration, and intensity of exercise in cirrhosis described in different research studies are shown in Table 2. Most of the studies included patients 40-50 years of age with compensated cirrhosis (i.e., Child-Pugh A) and the majority of the patients received specialized nutritional therapy together with the exercise program. Practically all the physical exercise protocols employed 2 forms of aerobic exercise: cycle ergometry and walking (utilizing a treadmill, or in one case, a pedometer, as the monitoring device).
Studies on exercise in cirrhosis of the liver.
| Author | Type of study | Intervention | N | Exercise description | Results | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | Exercise | Acute/chronic | Type (aerobic/anaerobic) | Warm-up (min) | Exercise(min) | Cool-down(min) | Workload | Days | Monitoring | ||||
| García-Pagan, et al.19961 | CT | 8 | Acute | Aerobic:Cycle ergometry | 8-10 | 30 and 50% | Cycle ergometry (E. Jaeger, Würz burg, Germany) | -↑ HVPG (p=<0.01)-↓ hepatic blood flow (p=<0.05) | |||||
| Saló et al., 199737 | CT | Ascites | 21 | Acute | Aerobic:Cycle ergometry | 30 | 3 METs | Cardiac Stress Testing Table, EDC, Inc., Lowell, MA | -↑ MBP (p=<0.0001)-↑ Heart rate (p=<0.0001)-↑ Plasma renin (p=0.001)-↑ Aldosterone (p=0.018)-↑ Norepinephrine (p=0.0001)-↓ RPF (p=0.0001)-↓ GFR (p=0.025)-↓ UNaE (p=0.032) | ||||
| Bandi JC, et al.19982 | Double-blind RCT | Propranolol/placebo | 11 | 12 | Acute | Aerobic: Cycle ergometry | 8-12 | 30%40 W | Cycle ergometer (Ergometry System 380, Siemens-Elema, Schönander, Sweden) | -↓ HVPG (p=<0.01) with propranolol-↑ HVPG (p=<0.01)-↓ HBF (p=<0.01) with placebo | |||
| Pattullo et al., 201310 | CT | Nutritional therapy | 10 HCV 9 CL | Walking | 10,000 steps | 7 days/week24 weeks | Pedometer (Digiwalker SW-700, Yamax, Tokyo, Japan) | -↑ Steps/day (p=0.001)-↓ Calorie intake (p=<0.001)-↓ BMI (p=<0.001)-↓ HOMA-IR (p=0.002)-↓ Insulin resistance (p=0.008) | |||||
| Zenith et al.201428 | RCT | 10 | 9 | Chronic | Aerobic:Cycle ergometry | 5 | 30(↑ 2.5 each week) | 5 | 60-80% of VO2 | 3 days/week8 weeks | Monark cycle ergometer (Monark Exercise AB, Vansbro, Sweden) | -↑ VO2 peak (p=0.001)-↑ MM (p=0.001),-Improved QoL (p=0.01) | |
| Román, et al.201438 | RCT | Leucine 10g/day | 9 | 8 | Chronic | Cycle ergometry | 10 | 10-30 | 10-15 | 60-70% MHR | 3 days/week6 weeks | - | -↑ Exercise capacity (p=0.01)-↑ MM (p=0.02)-Improved QoL (p=0.03) |
| Treadmill walking | 10 | 15-20 | 10-15 | 3 days/week6 weeks | |||||||||
| Resistance: Weights and elastic bands on arms | 5-10 | 3 days/weekLast 6 weeks | |||||||||||
| Macías- Rodríguez et al., 20167 | RCT | Nutritional therapy | 12 | 13 | Chronic | Cycle ergometry and kinesiotherapy | 10 | 40 | 10 | 60-80% MHR12-14 Borg rating | 3 days/week14 weeks | Cycle ergometer (Ergoline, Bitz, Germany) | -↓ HVPG (p=0.05)-Improved ventilatory efficiency (p=0.03) and PA in SPE |
| Debette et al., 201527 | CT | Therapeutic education: benefits of exercise | 8 | Chronic | Cycle ergometry | 20 | 2 days/week12 weeks | Cycle ergometer (DX1 ergometer Kettler, Germany) | -↑ VO2 peak (p=<0.008)-↑ maximum strength (p=0.02)-↑ distance of 6min walk (p=<0.02)ventilatory threshold (p=0.02)muscle strength (p=0.008) | ||||
| Bench step for strength | 20 | 70-80% MR3 series, 8 repetitions+1 repetition at week 6 | |||||||||||
| Román et al., 20169 | RCT | Relaxation | 9 | 14 | Chronic | Cycle ergometry and treadmill walking | 10 | 10-30 at the end | 10-15 | 60-70% MHR | 3 days/week12 weeks | - | -↑ TTE (p=<0.001)-↑ VAT (p=0.009)-↓ TBF (p=0.003)-↓ Timed Up&Go test (p=0.02)-↑ MM (p=0.01)In the exercise group |
| Weights and elastic bands | 5-10 | ||||||||||||
| Berzigotti A, et al201739 | Cohortand prospective multicenter | Nutritional therapy | 50 | Chronic | Aerobic and strength routine | 10 | 40 | 10 | 10-12 Borg rating | 1 day/week16 weeks | -↓ HVPG (P=0.0001)-↓ Weight (>10%) (p=0.024) | ||
| Kruger, 201840 | RCT | Nutritional therapy | 20 | 20 | Chronic | Home exercise training | 8 weeks | Cycle ergometer | In the exercise group↑ distance of 6min. walk (p=<0.009)↑ VO2 peak (p=<0.02) | ||||
BMI: body mass index; CL: cirrhosis of the liver; CT: clinical trial; GFR: glomerular filtration rate; HBF: hepatic blood flow; HCV: hepatitis C virus; HVPG: hepatic venous pressure gradient; MBP: mean blood pressure; METs: metabolic equivalents; MHR: maximum heart rate; MM: muscle mass; MR: maximal repetitions; PA: physical activity; QoL: quality of life; RCT: randomized clinical trial; RPF: renal plasma flow; SPE: supervised physical exercise; TBF: total body fat; TET: total effort time; UNaE: urinary sodium excretion; VAT: ventilatory anaerobic threshold; VO2: volume of oxygen.
1 watt (W) costs approximately 10.3ml of O2/min.
1 metabolic equivalent is equivalent to the resting O2 uptake in a healthy 70kg man.
The total duration of the protocols varied from 30 to 60min and a mean of 3 times a week of supervised physical exercise, plus exercises at home. Some protocols included 3-phase exercise programs, with a warm-up phase, a main component phase, and a cool-down phase. To limit exercise-induced injury, and in accordance with the guidelines recommended for the normal population, the 3 or 4-phase focus appears to be the best for exercise prescription in patients with early-stage cirrhosis. Thus, it is necessary to know the general exercise prescription regimen in the non-cirrhotic population recommended in the recent guidelines.13
Guidelines for exercise prescription in the population with no cirrhosisEven though the general exercise prescription regimen of the ACSM provides a useful base for patients with cirrhosis, it should not be thought of as an obligatory formula and the fact that it was not made for that population must always be remembered. The physical training sessions include 4 components: 1) warm-up (5-10min), 2) conditioning (20-60min), 3) cool-down (5-10min), and 4) stretching (10min). The exercise program follows a general plan under the acronym, FITT (Frequency, Intensity, Time [duration], and Type [modality]). Current guidelines recommend a frequency of 5 days/week of aerobic exercise that is mild (30-40% HR reserve [resting HR−maximum HR]) or moderate (40-60% of the HR reserve) for 30-60min/day (≥ 150min/week) that involves the large muscle groups (walking, swimming, jogging, dancing, among others).
Recommendations for prescribing exercise in cirrhosisTaking into account the prescription guidelines in the non-cirrhotic population and the abovementioned clinical studies on exercise in cirrhosis, 4 points for exercise prescription in patients with cirrhosis should be considered: 1) evaluating them carefully to search for both general and cirrhosis-induced complications that can limit exercising, 2) designing and choosing the most adequate exercise program based on each patient's state of health, 3) setting a goal; 4) verifying the best tool available for the follow-up of patient progression.
With respect to the first point, the evaluation that patients should undergo before beginning to exercise, so that injury or complications derived from pre-existing complications, as well as cirrhosis-specific ones, are prevented was previously depicted.
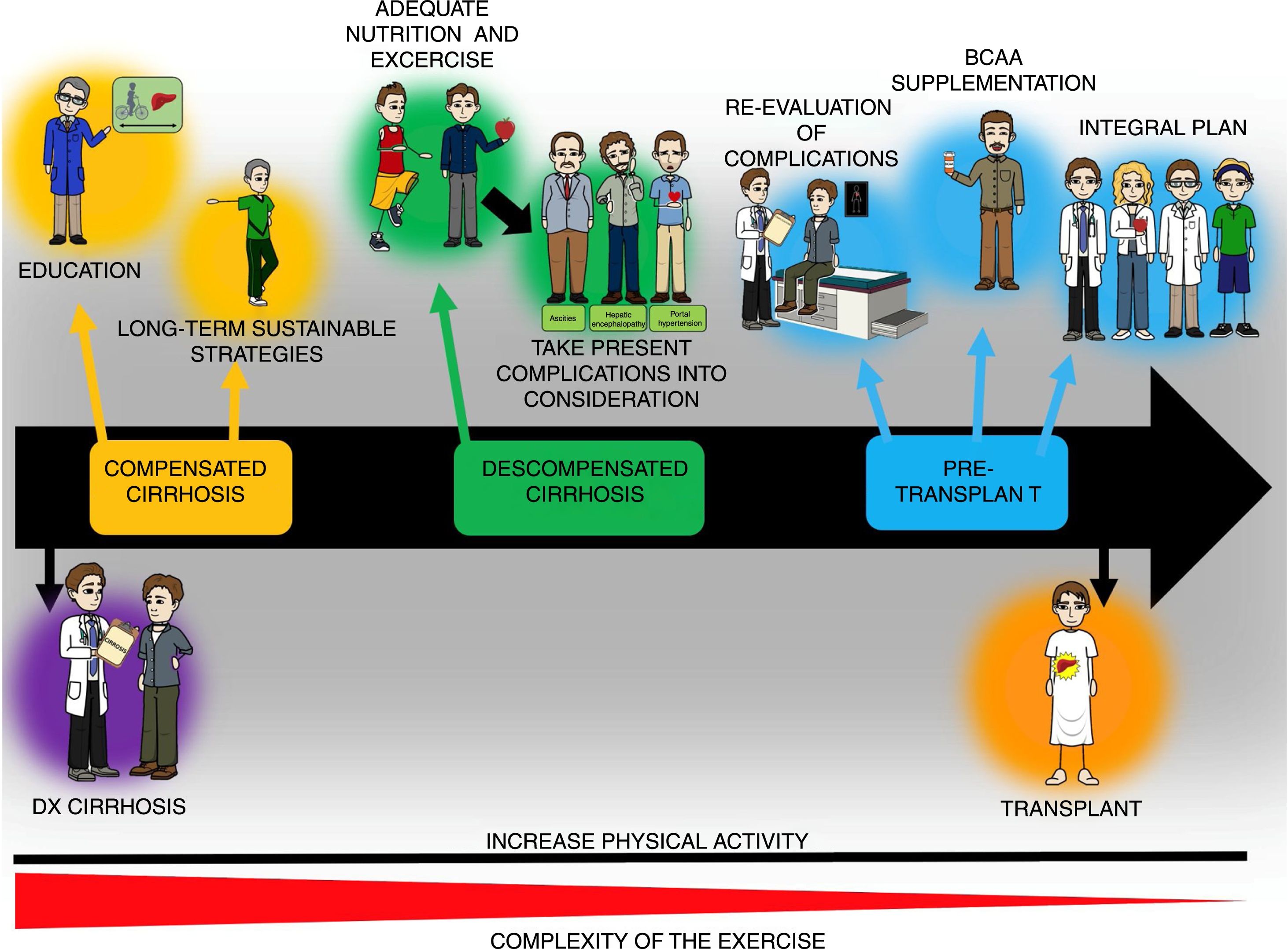
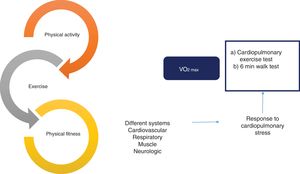
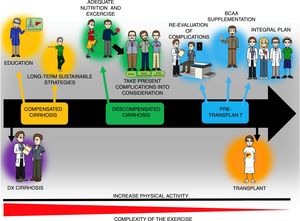
Once the state of health is assessed, the exercise prescription regimen described above with adaptations according to the studies on cirrhosis can be utilized. Stated in a simplified manner, the physical training session can consist of 3 components: 1) warm-up (5-10min), 2) main phase or physical conditioning (20-60min), and 3) cool-down and stretching (10min). During the warm-up and cool-down phases, movements that lubricate the most important joints that were or will be used are recommended, as well as stretching the muscles involved in the training session. In the main phase, in patients with poor physical condition, beginning with 20min of physical activity with gradual increases (5-10min every 1-2 weeks) is recommended (ACSM 10). Likewise, the FITT exercise program and the information available on the population with cirrhosis suggest 5 days/week of mild or moderate exercise (determined by the HRreserve [30-40%] or the Borg scale [10-14]) for 30-60min/day (≥ 150min/week) through an activity such as walking or cycle ergometry. In a practical manner, moderate physical activity of 150min/week is equivalent to≥5400-7900 steps/day. That may help guide the exercise prescription more easily.13 Finally, said model can be used to educate patients with cirrhosis, and at practically all stages of the disease, and thus serve as a base for post-transplant rehabilitation, consequently preventing the metabolic complications that can ensue (fig. 3).
General diagram of the exercise and nutrition intervention in patients with cirrhosis throughout the course of the disease. Increased physical activity is recommended throughout the course of the disease in patients with cirrhosis, even though the complexity of the exercise must be adjusted to the stage of the disease and the complications present at the time of evaluation. The exercise plan should include educating the patients as to its benefits and how to perform it, as well as adequate nutrition, which can require the use of supplements, such as the branched-chain amino acids (BCAAs). Finally, to achieve the goals of such a program, the participation of different specialists, including physicians, nutritionists, and physical activity experts, to adjust the program in accordance with disease stage.
In the patients in whom that program cannot be applied, such as patients confined to a wheelchair due to lack of physical activity, one of the first maneuvers would be to stand up and remain standing with help and gradually increase the standing time. Exercises for improving the muscles of the arm can also be added, such as repetitions with anti-stress balls. On the other hand, in patients with very early-stage cirrhosis (diagnosed through biopsy, with no sign of the disease), the exercise program is very similar to that for persons without cirrhosis, without neglecting the other limiting factors (cardiopulmonary, musculoskeletal, etc.). Patients with esophageal varices must follow the established management guidelines for that clinical entity and not limit physical activity only for said motive. In cases of ascites, the grade of that condition will determine the type and intensity of physical activity. Finally, patients with low-level hepatic encephalopathy should always be supervised when walking, to avoid injury (e.g., falls).
Having a clear goal before beginning the physical training programs aids in planning the rest of the points and in encouraging the patients and their families, so that the expected results can be obtained. For example, in the case of a patient with important malnutrition and candidate for a liver transplantation, the goal of improving the nutritional status (improving the quantity and quality of muscle tissue and the capacity of post-transplant cicatrization) will aid in having a well-structured plan and in carrying out adequate patient follow-up. (fig. 3).
The selection of the method for monitoring patient progress will depend on its availability at the hospital center, the goal of the program, the experience with its use (including reproducibility and reliability), costs, and whether it really evaluates that which requires monitoring or is a reliable surrogate.
Even though much information is still lacking with respect to exercise prescription in cirrhosis, the points described throughout the present article will provide an adequate basis for preventing the confinement of patients to a sedentary lifestyle and will facilitate having a clearer idea of the variables that must be considered in the clinical setting of cirrhosis. Finally, the increase in physical activity and exercise in the population with cirrhosis should begin with the education of the patient (disease, interventions, etc.) to achieve long-term sustainable strategies (fig. 3). Likewise, the complications of the patients must be considered when prescribing physical activity/exercise, as well as the general state of health, and nutritional therapy that is tailored to the needs of the patient must be implemented. Such a plan will help establish a routine in the patients that will even help prevent post-liver transplantation metabolic complications. The complexity of exercise/physical activity will change according to disease stage and should be considered in all patients with cirrhosis, but that, in itself, is not a contraindication for the intervention (fig. 3).
Nutritional therapyDietary management of patients with cirrhosis of the liver is an important aspect in the comprehensive treatment of the disease. In fact, different studies have shown that the combination of diet and physical exercise is more beneficial than either of the 2 interventions, alone.8,41
To establish a dietary plan in the patient with cirrhosis that exercises, the energy requirement based on disease stage, comorbidities, and the level of physical exercise to be prescribed must be calculated to so that there is sufficient energy intake to counteract the catabolism.
Energy requirementTo prescribe a successful nutritional therapy, it is essential that it fully covers the energy requirement of the patient. Said requirement can be calculated in two distinct manners. Under ideal conditions, it is obtained through methods, such as indirect calorimetry, but it is not very accessible due to its lack of availability and high cost. Therefore, different formulas and recommendations have been created to calculate basal energy expenditure (BEE). The available formulas that have been utilized in exercise interventions are shown in Table 3, along with the explanation of the populations in which they can be used.
Formulas for calculating basal energy expenditure (BEE).
| Name | Formula | Use | |
|---|---|---|---|
| Cunningham42 | General formula | BEE=[370+21.6 x lean muscle mass x 4.18] | Subjects with overweight or obesity.Critical patients.Greater precision in high-performance athletes. |
| Female | FFM=[79.5 – 0.24 (weight in kg) – 0.15 (age in years) x weight in kg/73.2] | ||
| Male | FFM=[69.8 – 0.26 (weight in kg) – 0.12 (age in years) x weight in kg/73.2] | ||
| Rapid calculation (Carrasco & Rojas)43 | Female | BEE=16.2kcal x weight in kg | Healthy subjects, with normal BMI, overweight, and obesity.Add stress factor due to disease and physical activity. |
| Male | BEE=17kcal x weight in kg | ||
| Rapid calculation ESPEN44 | TEE (with malnutrition)=35-40kcal x weight in kgTEE (without malnutrition)=30-35kcal x weight in kg | Stress factor is not added. | |
| Harris-Benedict45 | Female | BEE=655+(9.7 x weight in kg)+(1.8 x height in cm) - (4.7 x age in years) | Healthy subjects, with normal BMI.Patients with CL utilizing adjusted weight (Wilkens). |
| Male | BEE=66.5+(13.7 x weight in kg)+(5 x height in cm) - (6.8 x age in years) | ||
| IOM46 | Female | BEE=354 – 6.91+PA x [9.36 x weight (kg)+726 x height (m)] | Healthy subjects, with normal BMI.Commonly used in athletes. |
| Male | BEE=662 - 9.53+PA x [15.91 x weight (kg)+539.6 x height (m)] | ||
| Mifflin-St Jeor47 | Female | BEE=(10 x weight in kg)+(6.25 x height in cm) – (5 x age in years) - 161 | Subjects with overweight or obesity.Hospitalized patients. |
| Male | BEE=(10 x weight in kg)+(6.25 x height in cm) – (5 x age in years)+5 | ||
| WHO/FAO48 | Female | BEE 18-30 years=(14.7 x weight in kg)+496 BEE 30-60 years=(8.7 x weight in kg)+829 BEE> 60 years=(10.5 x weight in kg)+596 | Healthy subjects, with normal BMI and overweight. |
| Male | BEE 18-30 years=(15.3 x weight in kg)+679 BEE 30-60 years=(11.6 x weight in kg)+879 BEE> 60 years=(13.5 x weight in kg)+487 | ||
| Owen49 | Female | BEE=795+(7.18 x weight in kg) | Subjects with overweight or obesity.Validated in trained athletes. |
| Male | BEE=879+(10.2 x weight in kg) | ||
| Oxford48 | Female | BEE 18-30 years=(10.4 x weight in kg)+(615 x height in cm) - 282 BEE 30-60 years=(8.18 x weight in kg)+(502 x height in cm) – 11.6 BEE> 60 years=(8.52 x weight in kg)+(421 x height in cm)+10.7 | Healthy subjects, with normal BMI. |
| Male | BEE 18-30 years=(14.4 x weight in kg)+(313 x height in cm)+113 BEE 30-60 years=(11.4 x weight in kg)+(541 x height in cm) - 137 BEE> 60 years=(11.4 x weight in kg)+(541 x height in cm) - 256 | ||
| Schofield50 | Female | BEE 18-30 years=(0.062 x weight in kg)+2.036 BEE 30-60 years=(0.034 x weight in kg)+3.538 BEE>60 years=(0.038 x weight in kg)+2.755 | Healthy subjects, with normal BMI.Patients with elevated energy expenditure (burns).Older adult. |
| Male | BEE 18-30 years=(0.063 x weight in kg)+2.896 BEE 30-60 years=(0.048 x weight in kg)+3.653 BEE> 60 years=(0.049 x weight in kg)+2.459 | ||
| Valencia51 | Female | BEE 18-30 years=(11.02 x weight in kg)+679 BEE 30-60 years=(10.92 x weight in kg)+677 BEE> 60 years=(10.98 x weight in kg)+520 | Healthy subjects, with normal BMI, overweight, and obesity created in a Mexican population. |
| Male | BEE 18-30 years=(13.37 x weight in kg)+747 BEE 30-60 years=(13.08 x weight in kg)+693 BEE> 60 years=(14.21 x weight in kg)+429 |
FFM is calculated in the same way as in the Cunningham equation.
Sex (male=1/female=0).
B: burn (yes=1/no=0; CL: cirrhosis of the liver; EMV: expired minute volume FFM: fat-free mass; O: obesity; PA: physical activity; T: trauma; TEE: total energy expenditure.
Despite the fact that the Harris-Benedict and the rapid calculation formulas are the most widely used, the Cunningham, IOM, and Owen equations have been reported to be the most appropriate in athletes and subjects in interventions that involve physical exercise. Likewise, the Mifflin-St Jeor is the formula of choice in subjects with overweight or obesity because it favors gradual weight reduction.43,52 However, the choice of the formula for calculating the BEE of each patient depends on the criterion of the nutritionist in charge, who should consider the characteristics of each patient when making the calculation.
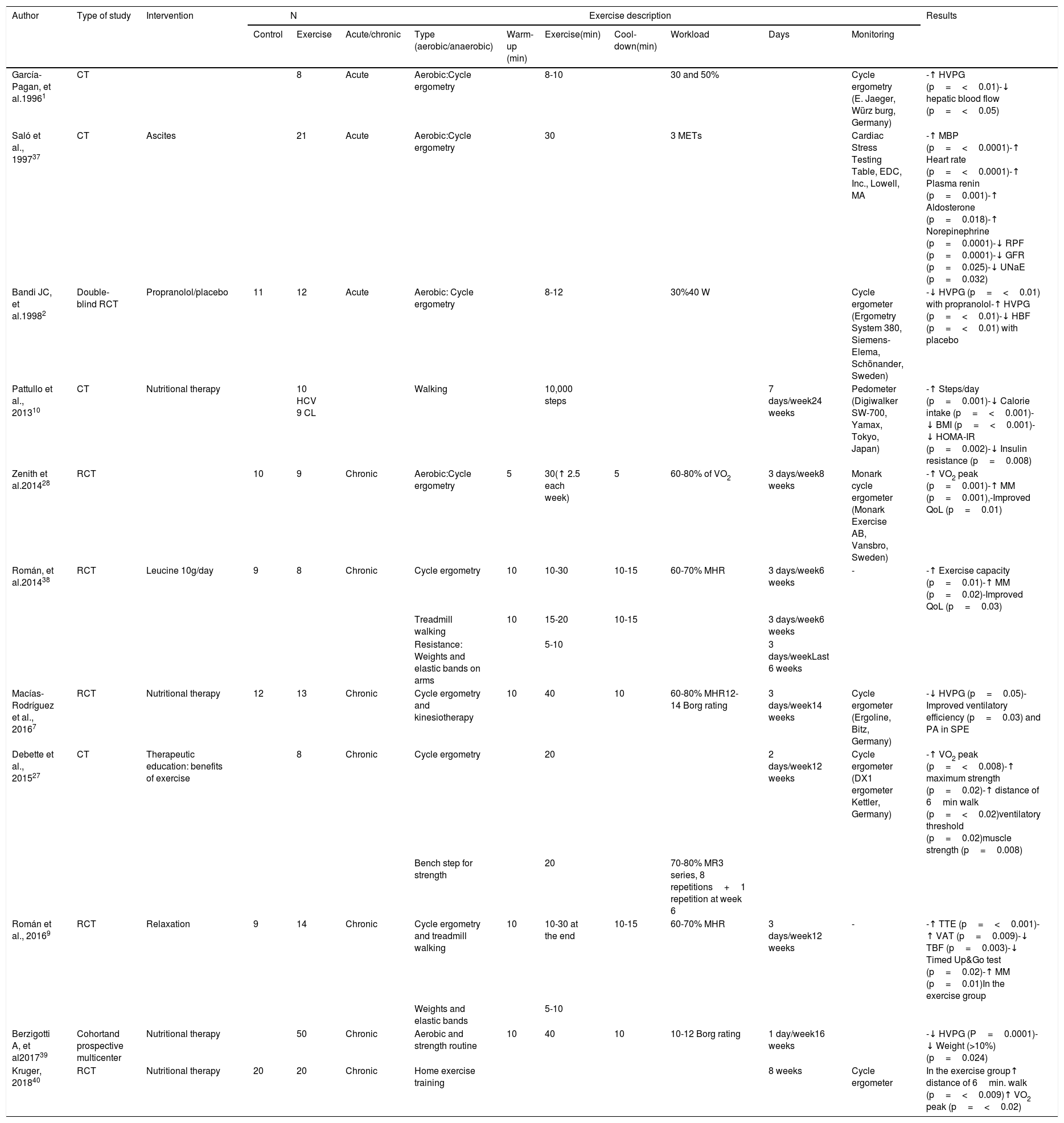
When the BEE is obtained from a rapid calculation formula, it is not necessary to add the stress factor due to disease or physical activity. However, if it is obtained using any other formula, the percentage of calories according to the disease stress factor is added to counteract the hypermetabolism present in up to 58% of patients, and the factor of the physical activity to be carried out must also be considered. (Table 4A and B).13,53,54
Hepatopathy stress factor.
| Stage | Percentage to add to the BEE |
|---|---|
| Mild fibrosis | - |
| Advanced fibrosis | 5% |
| Compensated cirrhosis | 10% |
| Decompensated cirrhosis (hepatic encephalopathy/esophageal varices/jaundice) | 15% |
| Decompensated cirrhosis (ascites/bacterial peritonitis/hepatocellular carcinoma) | 15-30% |
Thus, the total energy required is obtained from the BEE+the stage of hepatopathy+the level of physical activity to be indicated for the patient. Table 5
Macronutrient recommendation and distributionOnce the total energy in kilocalories is obtained, it is distributed between carbohydrates, proteins, and fats. The specific quantity of macronutrients depends on many factors, and include the amount of physical activity performed and the presence of complications.55
To begin the distribution, the quantity of proteins per kilogram of weight is first considered, based on the nutritional status of the patient:55
- •
No malnutrition: 1.2g/kg weight/day
- •
Mild malnutrition: 1.3g/kg weight/day
- •
Moderate malnutrition: 1.4g/kg weight/day
- •
Severe malnutrition: 1.5g/kg weight/day
The percentage of carbohydrates is then determined and can be from 45 to 65% of the total calorie intake. The percentage should be based on each patient, albeit a minimum carbohydrate intake of 55% is suggested in patients that perform physical exercise. The remaining calories should be received from the intake of fats. The patient should ingest at least 1ml for every kcal indicated in the diet. It is also important for the patient to maintain adequate hydration prior to and during physical exercise.44
A distribution of at least 5 meals that includes 2 snacks should be carried out. One snack can be indicated before performing the physical exercise and the other as an evening snack. In fact, even though the eating schedule depends on each patient, an interval between meals no greater than 4h is suggested, thus preventing long periods of fasting.
SupplementationSupplementation with BCAAs has been studied in the cirrhotic population and their consumption has been related to an increase in muscle mass, a decrease in episodes of hepatic encephalopathy, improved liver reserve in patients with hepatocellular carcinoma, reduced insulin resistance, and improved quality of life. Therefore, their consumption is widely supported in patients with chronic hepatopathy.56,57
In a pilot study, the effect of moderate aerobic exercise plus 10g of leucine daily was evaluated. There was improvement in functional capacity, quality of life, a significant increase in muscle mass, and no signs of complications in the group that received the intervention.38 The combined treatment of physical exercise plus supplementation with the 3 BCAAs has been evaluated in other studies and the results suggest that the combined intervention has a greater effect than treatment with supplementation or exercise alone. Improvement in anaerobic capacity and a potentially beneficial effect on glycemic control were observed. Supplementation with BCAAs has also has been related to lower incidence in the characteristic complications of the disease, but more evidence is still needed in relation to the benefits of supplementation in patients with cirrhosis.58
Another strategy that has been reported as beneficial is the implementation of an evening snack that provides 200-400kcal and is particularly rich in protein (20-40g), fiber, and complex carbohydrates, given that it has shown improvement in muscle mass and energy metabolism by covering the caloric needs during the night.59,60 In fact, when combined with BCAA supplementation, an evening snack has demonstrated important improvement in nutritional parameters, nitrogen balance, and quality of life.61
ConclusionsEven though there needs to be more evidence supporting the safety and efficacy of exercise under specific conditions in patients with cirrhosis and portal hypertension, currently available information supports its general use in that population. To adequately prescribe exercise, a priori knowledge of the general conditions of the patient is necessary, as well as the complications derived from cirrhosis. The goal that is expected to be reached through the exercise program should also be clear, as well as the existing limitations of the healthcare center.
Ethical disclosuresThe present research did not require patient informed consent because it is a literature review.
The present article did not require the authorization of ethics committees because it is a bibliographic review.
The authors declare that the present article contains no personal information that could identify the patients.
Financial disclosureNo financial support was received in relation to this article.
Conflict of interestThe authors declare that there is no conflict of interest.
Ricardo U. Macías-Rodríguez wishes to thank the Universidad Nacional Autónoma de México (UNAM), the Board of the INCMNSZ, and the Consejo Nacional de Ciencia y Tecnología (CONACYT), for their support in relation to the present work (part of the present article was based on work from the doctorate degree program carried out at the UNAM).
Please cite this article as: Macías-Rodríguez RU, Ruiz-Margáin A, Román-Calleja BM, Moreno-Tavarez E, Weber-Sangri L, González-Arellano MF, et al. Prescripción de ejercicio en pacientes con cirrosis: recomendaciones para la atención clínica. Revista de Gastroenterología de México. 2019;84:326–343.