In recent decades, Clostridium difficile infection (CDI) has become a worldwide health problem. Mexico is no exception, and therefore the Asociación Mexicana de Gastroenterología brought together a multidisciplinary group (gastroenterologists, endoscopists, internists, infectious disease specialists, and microbiologists) to carry out the “Consensus on the prevention, diagnosis, and treatment of Clostridium difficile infection”, establishing useful recommendations (in relation to the adult population) for the medical community.
Said recommendations are presented herein. Among them, it was recognized that CDI should be suspected in subjects with diarrhea that have a history of antibiotic and/or immunosuppressant use, but that it can also be a community-acquired infection. A 2-step diagnostic algorithm was proposed, in which a highly sensitive test, such as glutamate dehydrogenase (GDH), is first utilized, and if positive, confirmed by the detection of toxins through immunoassay or nucleic acid detection tests. Another recommendation was that CDI based on clinical evaluation be categorized as mild-moderate, severe, and complicated severe, given that such a classification enables better therapeutic decisions to be made. In mild-moderate CDI, oral vancomycin is the medication of choice, and metronidazole is recommended as an alternative treatment. In addition, fecal microbiota transplantation was recognized as an efficacious option in patients with recurrence or in the more severe cases of infection, and surgery should be reserved for patients with severe colitis (toxic megacolon), in whom all medical treatment has failed.
La infección por Clostridium difficile (ICD) en las últimas décadas se ha convertido en un problema de salud mundial. Nuestro país no es la excepción, y por ello la Asociación Mexicana de Gastroenterología reunió a un grupo multidisciplinario (gastroenterólogos, endoscopistas, internistas, infectólogos y microbiólogos), para que realizaran el «Consenso sobre prevención, diagnóstico y tratamiento de la infección por Clostridium difficile» y se establecieran recomendaciones (dirigidas a población adulta) de utilidad para la comunidad médica.
Las recomendaciones emitidas se presentan en este documento. Se reconoce que la ICD debe sospecharse en sujetos con diarrea con antecedente de uso de antibióticos y/o inmunosupresión, pero que también puede adquirirse en la comunidad. Se propone seguir un algoritmo de dos pasos para el diagnóstico, utilizando una primera prueba de alta sensibilidad como la glutamato deshidrogenasa (GDH) y, en caso de resultado positivo, se debe confirmar mediante detección de toxinas por técnica de inmunoensayo o con pruebas de detección de ácidos nucleicos. Se recomienda categorizar la ICD con base en la evaluación clínica en leve-moderada, grave o grave complicada, ya que esto permite una mejor decisión terapéutica. En la ICD leve-moderada la vancomicina oral es el medicamento de elección, y se recomienda usar metronidazol como tratamiento alternativo. El trasplante de microbiota fecal se reconoce como una opción eficaz ante las recurrencias o los casos más graves, y la cirugía debe reservarse a pacientes con colitis grave (megacolon tóxico) que han fallado a todo tratamiento médico.
Clostridium difficile infection (CDI) epidemiology has changed dramatically in recent decades and has become an important worldwide health problem. The appearance of increasingly more virulent strains, inadequate antibiotic use, and the aging of a population affected by a larger number of chronic and debilitating diseases are factors that have increased the incidence of CDI, with the consequent rise in morbidity, mortality, and healthcare costs. Thus, CDI has gone from being a strictly nosocomial infection to a problem that is frequently community-acquired, with a broad clinical presentation spectrum. Mexico is no exception and different studies on this health problem, some of which have been published in the Revista de Gastroenterología de México, have caught the attention of the medical community.
In October of 2017, the Asociación Mexicana de Gastroenterología brought together a multidisciplinary group of healthcare professionals made up of gastroenterologists, endoscopists, internists, infectious disease specialists, and microbiologists to carry out the “Consensus on the prevention, diagnosis, and treatment of Clostridium difficile infection”, establishing useful recommendations for the medical community. It is important to mention that the recommendations are intended for adult patients, and not the pediatric population.
The specific aim of the present consensus was to prepare an up-to-date document on the epidemiology, diagnosis, treatment, and prevention of CDI, for its practical application in Mexico. The recommendations included are based on an extensive review of the literature and on the consensus opinion of the participating specialists.
MethodsThe consensus was developed using the Delphi process as previously described.1 Three coordinators (ATAA, MRZS, JAVRV) and a general coordinator were designated and 18 experts in the specialties of Gastroenterology and Infectious Diseases were invited to participate. The coordinators carried out a detailed search utilizing the following databases: CENTRAL (The Cochrane Central Register of Controlled Trials), MEDLINE (PubMed), EMBASE (Ovid), LILACS, CINAHL, BioMed Central, and the World Health Organization International Clinical Trials Registry Platform (ICTRP). The search encompassed the time frame of January 1, 2008 to October 28, 2017 and its criteria included the term, “Clostridium difficile”, combined with the following terms: “epidemiology”, “incidence”, “prevalence”, “Mexico”, “pathophysiology”, “diarrhea”, “microbiota”, “diagnosis”, “differential diagnosis”, “treatment”, “antibiotics”, “therapy”, “management”, “review”, “guidelines”, and “meta-analysis” and their Spanish equivalents. The complete bibliography was made available to all the consensus members.
Afterwards, the coordinators formulated 55 statements that were submitted to a first anonymous, electronic round of voting (November 1 to 8, 2017) to evaluate the content and wording of the statements. The consensus participants cast their votes utilizing the following responses: a) in complete agreement, b) in partial agreement, c) uncertain, d) in partial disagreement, and e) in total disagreement.
After the first round of voting, the coordinators made the corresponding modifications. The statements that reached> 75% complete agreement responses were kept and those that had> 75% complete disagreement responses were eliminated. The statements whose complete agreement responses were ≤ 75% and whose complete disagreement responses were ≤ 75% were reviewed and restructured. The revised statements underwent a second round of anonymous, electronic voting (December 1 to 8, 2017). Based on the comments from the second round of voting, the newly revised statements underwent a third round of voting (January 16 to 23, 2018), where, in addition to writing each statement, the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) system was used to establish the strength of each recommendation and evaluate the quality of evidence sustaining it.2 In the GRADE system, the quality of evidence is not determined solely on the study design or methodology, but it is also judged on having a clearly posed question in relation to a clearly formulated outcome measure. [3] Based on those criteria, evidence is graded as high, moderate, low, or very low. In addition, the GRADE system establishes the strength of the recommendations as strong or weak, in favor of or against the statement or intervention. It is important to mention that the GRADE system was used in regard to diagnostic tests and therapeutic interventions. As shown in Table 1, the GRADE system employs a code that uses upper case letters to describe the quality of evidence, followed by a number to indicate the strength of the recommendation in favor of or against a statement or intervention.
GRADE system codes.
| Quality of evidence | Code |
| • High | A |
| • Moderate | B |
| • Low | C |
| • Very low | D |
| Strength of the recommendation | |
| • Strong, in favor of the statement or intervention | 1 |
| • Weak, in favor of the statement or intervention | 2 |
| • Weak, against the statement of intervention | 2 |
| • Strong, against the statement or intervention | 1 |
Adapted from Oñate-Ocaña and Ochoa-Carrillo.3
The results of the third round of voting were presented on January 31, 2018, at a face-to-face meeting that took place at the offices of the Asociación Mexicana de Gastroenterología in Mexico City. At that meeting, the statements with agreement> 75% were ratified. Those that did not reach 75% agreement in the previous voting rounds were discussed and voted on in a final effort to reach a consensus, and if that did not occur, they were eliminated. Once all the statements of the consensus were established, the coordinators formulated the present manuscript, which was reviewed and approved by all the members of the consensus group.
ResultsThe coordinators initially proposed 55 statements. In the first round of voting, 24 statements (44%) were eliminated because no consensus was reached in reference to them. The second round of voting was carried out on 31 statements, and according to its results, 35 statements were presented for the third round of voting. At the face-to-face meeting, a total of 32 statements were presented, 26 (82%) to be ratified and 6 (18%) to be voted on again. At the end of the face-to-face meeting, a total of 27 statements remained, once several statements were revised, eliminated, or joined together. The final statements and voting results follow below.
General aspects, epidemiology, and risk factors- 1.
In recent decades, an increase in the incidence of Clostridium difficile infection has been reported in numerous populations, including both hospital and community environments
Agreement reached: 91% in complete agreement, 9% in partial agreement
Since the 1970s, CDI has been associated with antibiotic use and it is considered a nosocomial pathogen. CDI presents in up to 8% of hospitalized patients and is the most common cause of nosocomial diarrhea worldwide. [4]6 It is the causal agent of 15-25% of the cases of antibiotic-associated diarrhea, 50-75% of the cases of antibiotic-associated colitis, and 90-100% of the cases of antibiotic-associated pseudomembranous colitis.5–7 Its incidence has increased dramatically since the year 2000.8 Its epidemiology has been modified in the last two decades due to outbreaks in confined settings of long-term care, such as nursing homes, as well as in the community.8,9 In addition, the Emerging Infections Program of the Centers for Disease Control and Prevention (CDC) discovered that nearly 50% of all initial cases of CDI had begun in the community.8 In Mexico, in a retrospective study conducted at 4 hospitals from 3 different cities, the authors reported that of the 487 cases of CDI included in the study, 43 (8.8%) were diagnosed in 2012 and 22 (4.5%) in 2013. There was an important increase in 2014, with 121 cases (24.8%) and in 2015, with 301 cases (61.8%).10 Recently, in a case-control study conducted at a tertiary care hospital center in Mexico City (2015 and 2016), Ochoa-Hein et al. reported that of the 329 cases observed within the study period, 155 (47.1%) were new hospital-acquired cases and 37 (11.2%) were community-acquired cases.11
The increase in more severe cases has been associated with a rise in more virulent strains (hypervirulent strains), such as the NAP1/BI/027 strain, which is the North American pulsed-field gel electrophoresis type 1 (NAP1), restriction endonuclease analysis group BI, and PCR ribotype 027.8,12 That strain has been associated with higher recurrence and mortality rates in the United States, Canada, the United Kingdom, and Europe.8,13 For example, a study that included 15,461 cases of CDI, showed a significant increase in the frequency of the NAP1/BI/027 strain in the cases related to hospital care, compared with community-acquired cases (30.7% vs. 18.8%, p <0.001).14
In Mexico, the prevalence of the NAP1/BI/027 strain varies from 28 to 91%.10,14–16 In a 2015 study on 22 patients, Camacho-Ortiz et al.15 demonstrated that 91% of the cases were positive for the NAP1/BI/027 strain. In another study, Morfín-Otero et al.16 examined the outbreaks of CDI at a tertiary care hospital over a 12-month period (n=288), and the NAP1/BI/027 strain was identified in 31 (39%) of the confirmed CDI cases, presenting more frequently in patients with a history of quinolone prescription (39.13 vs 10.4%, p=0.03) and relapses (19 vs 4%, p=0.03). In the study by Dávila et al.10 (n=487), 51.1% of the cases corresponded to the NAP1/BI/027 strain of C. difficile, whereas in a more recent study, that strain was reported in 28% of the cases.17 The clinical significance of the NAP1/BI/027 strain in the Mexican population is uncertain. For example, in an observational study conducted at a tertiary care hospital center within the time frame of 2008 and 2015, Tamez-Torres et al.17 reported a similar outcome in episodes caused by the NAP1/BI/027 strain, when compared with those caused by other ribotypes.
- 2.
The CDI spectrum is variable and ranges from mild diarrhea to severe complications, such as pseudomembranous colitis, toxic megacolon (fulminant colitis), sepsis, and death
Agreement reached: 91% in complete agreement, 9% in partial agreement
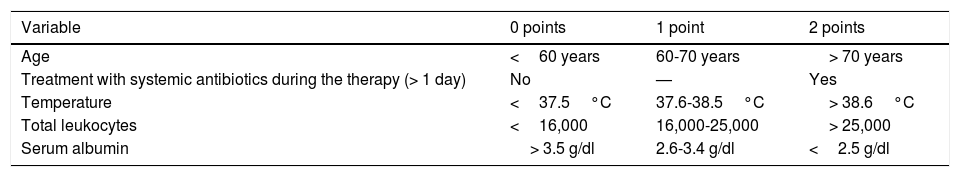
In CDI presenting with a mild clinical course, diarrhea18 is mild-moderate19 (> 3 and <10 bowel movements per day), accompanied by cramping, but rarely by fever or general malaise. Even though C. difficile toxins are now detectable in those cases, endoscopy and histology can be normal.20 When there are more than 10 bowel movements in 24h, CDI is considered severe.19 It can present with mild-to-moderate abdominal pain, nausea, anorexia, dehydration, fever, and leukocytosis. Sigmoidoscopy reveals erythematous regions that are diffuse or in patches, but with no membranes.20 Importantly, up to 20% of the patients with severe CDI can present with ileus and not have diarrhea. Some authors have defined severity based on clinical and laboratory findings.18,21,22 ATLAS is a scoring system for severe CDI that combines clinical and laboratory variables (Table 2) and is useful for patient stratification.23
ATLAS registry system and score for each variable22
| Variable | 0 points | 1 point | 2 points |
|---|---|---|---|
| Age | <60 years | 60-70 years | > 70 years |
| Treatment with systemic antibiotics during the therapy (> 1 day) | No | — | Yes |
| Temperature | <37.5°C | 37.6-38.5°C | > 38.6°C |
| Total leukocytes | <16,000 | 16,000-25,000 | > 25,000 |
| Serum albumin | > 3.5 g/dl | 2.6-3.4 g/dl | <2.5 g/dl |
Pseudomembranous colitis is considered a typical manifestation of CDI and presents in 40-60% of the cases.12 In that variety, diarrhea is generally profuse, with moderate-to-severe abdominal pain. Colonoscopy reveals yellowish plaques. The left colon is affected in the majority of patients, but the pathology can involve the entire colon. In Mexico, Velarde-Ruiz Velasco et al.12 reported that pseudomembranous colitis had a certain tendency to present more frequently with the hypervirulent strain (47% vs. 28%), but those results showed no statistically significant difference (RR: 1.62, p=0.23).
When CDI symptoms are severe and the colon is shown to be distended (> 6cm in diameter in the transverse colon) in radiologic studies, the disease is considered “toxic megacolon”.17 A severe systemic inflammatory response associated with toxic megacolon is known as fulminant colitis (see statement 8).
- 3.
Colonization by Clostridium difficile is more frequent in patients with a history of hospitalization within the previous 2 months, immunosuppression, and the use of chemotherapy, antibiotics, proton pump inhibitors, or H2 antagonists
Agreement reached: 83% in complete agreement, 17% in partial agreement
There is no consistent definition of colonization by C. difficile. However, according to a recent review, colonization is considered when the bacillus is detected and there are no signs or symptoms of infection.24 The subjects are most likely protected from progression to infection by a humoral response to clostridial toxins,24 but those individuals act as a reservoir of infection and may present a risk for others.25
The prevalence of C. difficile colonization varies among different groups. It ranges from 0 to 15% in healthy individuals, from 18 to 90% in neonates and breast-feeding infants, from 0 to 54% in the elderly in nursing homes, and from 0 to 13% in healthcare workers.24C. difficile screening to detect colonized individuals should not be a routine procedure because those subjects are not given treatment.24,26
Even though the exact mechanism of colonization by C. difficile is unknown, spores have been detected in the environment (water, potable water, pools, soil), in intermediary hosts (animals), and in food (pork, beef, turkey, seafood, and ready-to-eat vegetables).5 Nevertheless, there are no reports of outbreaks after eating foods contaminated with the pathogen.27 Humans probably ingest C. difficile spores frequently, but the majority remain asymptomatic and uncolonized if their gut microbiota is intact.5,28C. difficile spores resist the acid environment of the stomach and germinate in the intestine.6,12,29 Primary bile acids in the duodenum are considered facilitators of the germination process.27 Under normal conditions, the gut microbiota impedes colonization by pathogens through different mechanisms, such as stimulation of the host immune system, nutrient depletion, and inhibition through bacteriocins.13,27
The association between proton pump inhibitors (PPIs) and CDI has been observed in recent years and prolonged gastric acid suppression is assumed to be the most important mechanism. Even though that association is controversial, a rational prescription of PPIs is recommended.30,31
- 4.
CDI should be suspected in a patient with diarrhea and a history of antibiotic use and/or immunosuppression, whose diarrhea is community-acquired, or presents after more than 48h of hospitalization, or within 8 weeks after hospital release
Agreement reached: 87% in complete agreement, 9% in partial agreement, 4% uncertain
CDI is defined as the presence of diarrhea (3 or more unformed stools in 24h) and a stool test that is positive for C. difficile toxins, toxigenic C. difficile detection, or the presence of pseudomembranous colitis demonstrated through colonoscopy.24
In most patients with CDI, there is a history of treatment with antimicrobial agents within the past 8 weeks or immunosuppression.26 The definition of community-acquired CDI has varied over time, but currently, and in line with the epidemiologic classifications of CDI according to Lessa et al.32 and Martin et al.,28 the temporality parameters in the different clinical settings are:
- •
Community-acquired. A confirmed CDI case with positive specimen collection in the outpatient setting or ≤ 3 days after hospital admission, in a patient that was not in a hospital center during the previous 12 weeks. Community-acquired cases have a frequency of 10 to 30% and generally affect younger patients, compared with hospital-acquired cases.28
- •
Community-onset presentation associated with hospital care. A confirmed CDI case with positive specimen collection in the outpatient setting or ≤ 3 days after hospital admission from a private residence, with a history of overnight stay at a hospital center (or nursing home) within the previous 12 weeks.
- •
Hospital-acquired. A confirmed CDI case with positive specimen collection> 3 days after hospital admission.
- •
Nursing home-onset or at another confined setting of long-term care: a confirmed CDI case with positive specimen collection at the confined setting facility or within the first days of hospital admission from said facility. In some countries, confined setting facilities, such as nursing homes, are considered hospital centers.
Antibiotic use is regarded as the main risk factor for an individual to develop CDI. The mechanism proposed is dysbiosis and the antibiotics considered to be of greater risk are: clindamycin, fluoroquinolones, third-generation cephalosporins, and aminopenicillins.33,34 Aminoglycosides, beta-lactamase inhibitors, carbapenems, doxycycline, linezolid, macrolides, rifampicin, rifaximin, and tigecycline are considered low-risk agents.
According to a systematic review and meta-analysis that included 12 studies (n=56,776 patients), the authors found that community-acquired CDI was associated with antibiotic and corticosteroid use, in addition to inflammatory bowel disease (IBD), hematologic neoplasia, kidney failure, and diabetes mellitus.35
- 5.
CDI recurrence is defined by the presence of a second episode within 8 weeks after the initial episode that was clinically resolved through treatment.
Agreement reached: 96% in complete agreement, 4% in partial agreement
After an episode of CDI, between 10 and 30% of the patients present with recurrence, defined as the appearance of a second episode with 8 weeks after the first one.20,21 There is a 40% risk for a second recurrence and a 50% risk for a third relapse.21
Recurrent CDI may be due to remnants of spores germinating in the colon when antibiotic treatment has ceased or to community-acquired reinfection.13,20 The risk factors for CDI recurrence are: age ≥ 65 years, additional antibiotics during follow-up, PPI use, renal insufficiency, recent or prolonged hospital stay, or CDI caused by the NAP1/BI/027 strain.5,36
- 6.
A greater risk for CDI has been observed in advanced-age patients, in patients with immunosuppression, in patients that have received antibiotics, in patients with inflammatory bowel disease, hospitalized patients, and residents of long-term care or confined-setting facilities
Agreement reached: 91% in complete agreement, 9% in partial agreement
Risk factors for CDI can be divided into 3 groups:
- •
Environmental factors. Hospital stay or prolonged or confined stay in facilities such as nursing homes.4,5,13
- •
Host factors. Age above 65 years, IBD, immunodeficiency, hematologic neoplasias, malnutrition, and decreased serum albumin levels.4,5,13 Patients with IBD (ulcerative colitis or Crohn's disease) have a 4.8-fold higher risk for CDI,37 especially those exposed to infliximab, steroids, adalimumab, or metronidazole, and those with other comorbidities.37 Furthermore, CDI complicates the course of IBD and is associated with longer hospital stay, a higher colectomy rate, and greater mortality.38 Malignant neoplasias, chemotherapy, and human immunodeficiency virus infection favor immunosuppression and are also associated with CDI.13 Of the oncology patients, women with breast cancer present with a higher risk for CDI. Post-solid organ transplantation patients have a 5-fold increased risk for hospital-acquired CDI, compared with other hospitalized patients.39 As described in statement 3, the association between PPIs and CDI is controversial.40 A history of appendectomy, nasogastric tube placement, and abdominal surgery are also subjects of debate in relation to the development of CDI.13
- •
Factors that induce dysbiosis. As described in statement 4, antibiotic use is the main predisposing factor to the development of CDI.
- 7.
Age (above 65 years), cancer, cognitive deterioration, and cardiovascular, respiratory, and renal comorbidities are risk factors that have been associated with greater morbidity and mortality due to CDI
Agreement reached: 96% in complete agreement, 4% in partial agreement
CDI-associated morbidity includes the performance of colectomy, recurrent CDI, hospital release to confined-setting facilities, and hospital readmission. Mortality attributed to CDI was thought to be under 1.5%, but since 2000, it has increased to 4.5-5.7%, reaching rates of 6.9-16.7% during epidemic periods.21
Pre-existing cardiovascular diseases (ischemic heart disease, heart failure), respiratory insufficiency, and chronic lung and kidney diseases are associated with a higher mortality rate. In a cohort study conducted in the United Kingdom that included 2,761 patients, the risk for comorbidity-related mortality at 30 days in patients with CDI was evaluated. Kidney disease was the main predictor of death. Having some type of cancer was a predictor of death starting at 60 years of age.31 Liver disease and cognitive deterioration were considered predictors of severe disease, but not of death.40,41
- 8.
The clinical predictors for the development of fulminant colitis due to Clostridium difficile are age (above 70 years), hemodynamic instability, hypoalbuminemia, leukocytosis (above 18,000/mm3), inflammatory bowel disease, and antiperistaltic medication use.
Agreement reached: 96% in complete agreement, 4% in partial agreement
The progression of CDI to fulminant colitis is rare (1-3%), but the mortality rate is considerable in that group of patients due to the progression to toxic megacolon and colonic perforation.20,21,41 The importance of diagnosing fulminant colitis lies in the fact that 0.5 to 2.6% of those patients require colectomy42 and the associated mortality rate is 35-80%.41
In general, signs of systemic inflammatory response and hemodynamic instability, serum creatinine levels> 1.5-fold higher than the baseline value (or serum creatinine ≥ 1.5mg/dl and leukocytosis> 18,000/mm3), antiperistaltic medication use (such as narcotics and anticholinergics), elevated serum lactate, surgical intervention within the previous 30 days, and aggregated IBD are considered poor outcome factors.41–43
Palau-Dávila et al.44 identified risk factors in Hispanic hospitalized patients. They found the following risk factors for colectomy: a Charlson index ≥ 1, ICU stay, and ≥ 4 days with diarrhea prior to CDI diagnosis. The associated factors for general mortality within the first 30 days were: low albumin levels (< 2.00g/dl) and treatment failure.42
Diagnosis- 9.
CDI should be diagnosed only when there is clinical suspicion based on the 2-step algorithm
Quality of evidence: A1
Strength of the recommendation: strong, in favor of
Agreement reached: 100% in complete agreement
The exact mechanism through which C. difficile causes a symptomatic infection is not completely understood, but we know that the microorganism is not invasive, and that toxin production is the key element of the pathogenesis. Laboratory tests, alone, cannot distinguish between asymptomatic colonization and clinical symptoms of infection. Therefore, diagnostic tests should be carried out only in patients in whom there is clinical suspicion (symptomatic patients).45,46
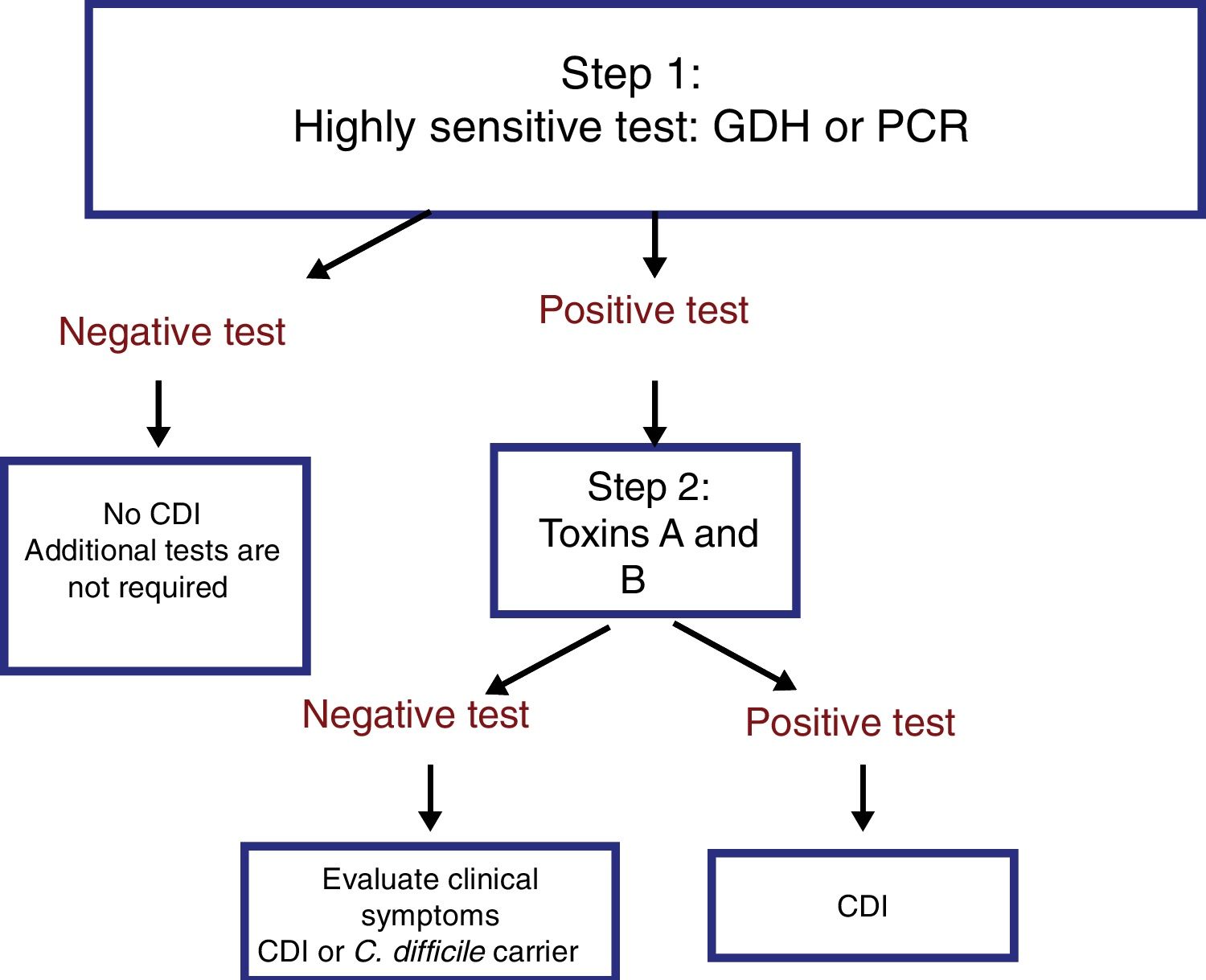
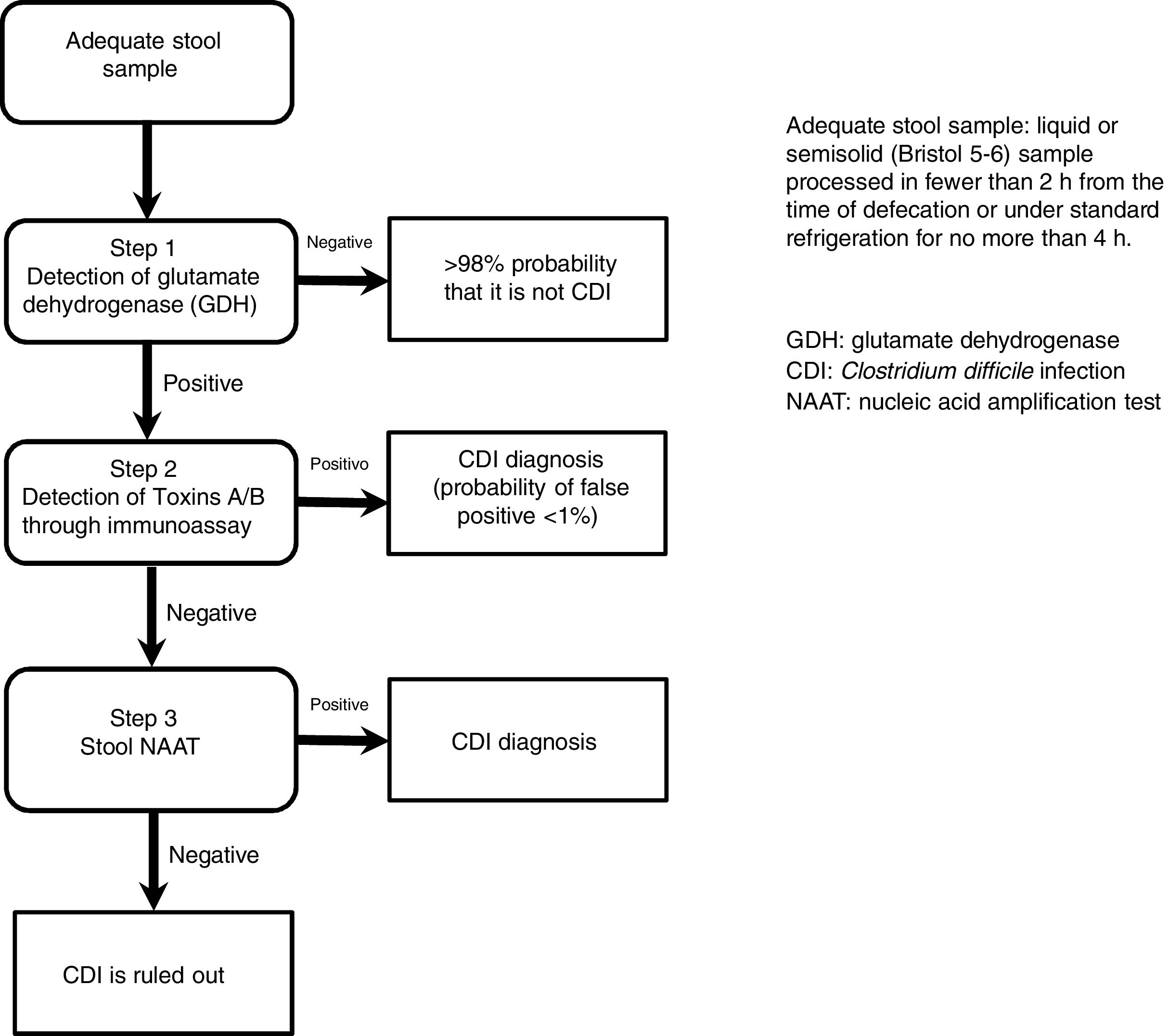
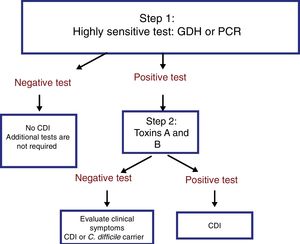
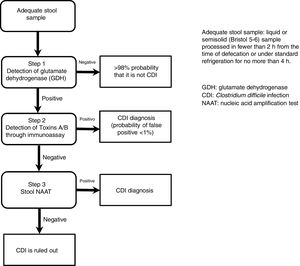
The most useful method for making the diagnosis when there is clinical suspicion of CDI is the 2-step algorithm (fig. 1). A first high-sensitivity test, such as glutamate dehydrogenase (GDH) or nucleic acid amplification tests (NAATs) through the polymerase chain reaction (PCR) technique should be performed. In the case of a positive result, diagnosis should be confirmed through immunoassay for toxin detection.47–49 The consensus group proposes a 2-step diagnostic algorithm. The multi-step algorithm that has recently been suggested in articles and international guidelines (fig. 2) can be useful in a small group of patients in whom there is diagnostic suspicion but no toxin detection and can only be applied in exceptional cases due to its high cost.47–49
GDH is an enzyme that is produced and secreted by C. difficile in large quantities, compared with toxins A and B, making its detection adequate and highly sensitive for diagnosis. The cost of the test is generally accessible, making it an ideal test for the initial approach in patients with diarrhea of recent onset and with risk factors for CDI.47 The authors of a meta-analysis reported that the diagnostic accuracy of GDH for C. difficile in stool was high, and when compared with the toxigenic culture, reached a sensitivity and specificity> 90%.50 GDH has a specificity of 80-100%, given that it detects toxigenic and nontoxigenic strains of the organism.
The new-generation NAATs detect and amplify pathogen-specific DNA or RNA sequences. The advantages of the NAATs include high sensitivity, high specificity, and velocity. Because viable cells are not needed, the aspects of sampling, manipulation, transport, and storage are simplified. Cultures are not required either. The NAATs can play a supportive role in the diagnostic process of CDI as part of the 2 or 3-step algorithm.47,51
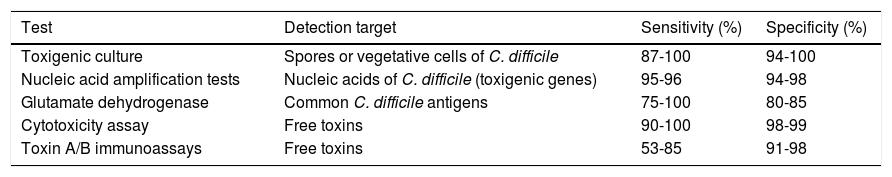
The toxigenic culture is still considered the gold standard for the diagnosis of CDI.52 It is used as the reference test for comparing the other diagnostic tests, reporting variable sensitivity and specificity for toxin detection.50 The diagnostic yield of the cell cytotoxicity neutralization assay (CCNA) has been reported to be similar to that of toxigenic culture. In that assay, a filtrate from a recently obtained stool simple is inoculated onto several sensitive cell lines to evaluate the cytopathic effect of the C. difficile toxins, particularly toxin B (TcdB). The CCNA has 90.8% sensitivity and 98.3% specificity.47,53 Sensitivity and specificity of the different diagnostic tests for CDI are variable and they are shown in Table 3.
Characteristics of the different diagnostic tests for Clostridium difficile infection45–50
| Test | Detection target | Sensitivity (%) | Specificity (%) |
|---|---|---|---|
| Toxigenic culture | Spores or vegetative cells of C. difficile | 87-100 | 94-100 |
| Nucleic acid amplification tests | Nucleic acids of C. difficile (toxigenic genes) | 95-96 | 94-98 |
| Glutamate dehydrogenase | Common C. difficile antigens | 75-100 | 80-85 |
| Cytotoxicity assay | Free toxins | 90-100 | 98-99 |
| Toxin A/B immunoassays | Free toxins | 53-85 | 91-98 |
It is important to emphasize that the 2-step algorithm is the most adequate because: 1) it prevents unnecessary treatment and its consequences, and 2) an initial evaluation with an inexpensive GDH test enables the rapid identification of negative samples, limiting the use of the costlier NAATs to those samples that are positive for GDH.47,48
- 10.
The diagnostic tests for CDI should only be performed on patients with diarrheic stools that present with risk factors. The exception are patients with ileus, in whom a rectal swab for carrying out PCR can be useful
Quality of evidence and strength of the recommendation:
Patients with risk factors: A1, strong, in favor of
Patients with ileus: C1, strong in favor of
Agreement reached: 100% in complete agreement
The medical personnel can increase the efficacy of the diagnostic tests by ordering them in patients that have the clinical probability of presenting with CDI. They should not be routinely requested in patients that have taken a laxative within the past 48h and specificity can also be increased by rejecting non-diarrheic stool (Bristol 1-5).18,47,54 If a patient has diarrhea that is not clearly attributable to underlying conditions (IBD, irritable bowel syndrome [IBS], post-infectious IBS, enteral nutrition, chemotherapy, or laxatives), then diagnostic tests for CDI are indicated, and even more so if the patient presents with the risk factors described in statement 6.47 In the minority of patients in whom CDI occurs as ileus and the collection of stool is complicated, the American College of Gastroenterology recommends the use of a “rectal swab” to collect a stool sample and the performance of a NAAT.47 Said recommendation is based on a prospective study in which 95.7% sensitivity and 100% specificity for the diagnosis of CDI through a rectal swab was reported.55
- 11.
Toxin A and B determination through the immunoassay technique has variable sensitivity. A negative test does not exclude the diagnosis
Quality of evidence: B1
Strength of the recommendation: strong, in favor of
Agreement reached: 78% in complete agreement, 22% in partial agreement
Since the identification in the 1970s of the toxin that produces C. difficile, several diagnostic methods for detecting the infection have been used. The majority of tests are based on the detection of the C. difficile toxin. Thus, it is important to remember that the pathogenic process begins with the germination of CD spores and the multiplication of the vegetative forms, whereas the second phase of the pathogenic process is the production of toxins. The strains of clinical interest are those that produce the toxins: A (TcdA) or B (TcdB) or both. Toxins A and B are encoded by the tcdA and tcdB genes that are located on the PaLoc locus (19.6kb).48,56 Traditionally, toxin A was considered enterotoxic and toxin B cytotoxic, but both are cytotoxic for different cell types and they induce an increase in vascular permeability and cause bleeding. In addition, the two toxins act synergically in the destruction of digestive tract cells. Nevertheless, the individual role of each of the toxins is still a subject of debate.5,47,48]7–59
There are numerous commercial tests for C. difficile that have different detection objectives: the toxin immunoassay analysis (ELISA) and CCNA (membrane and toxin enzyme immunoassay [EIA]) detect free toxins and the GDH test and NAATs detect the presence of all toxigenic C. difficile strains.56 For several years, C. difficile toxin determination through an immunoassay technique was an economic, rapid, and easy-to-perform analysis, and so those methods were the most common for diagnosing CDI in the majority of laboratories.60,61 Because some C. difficile strains do not produce toxin A, the performance of toxin immunoassays that detect both A and B toxins is recommended. Stool samples can be kept at 4° C for days or weeks before their analysis for toxin detection without losing their diagnostic capacity.62
Variable sensitivity and specificity of toxin EIAs have been reported. Sensitivity varies from 58 to 99% and specificity from 90 to 100%.6,51]3–72
The inconsistent sensitivity of the EIAs may be due to several factors, such as antigenic variation between the toxins from different circulating strains, inadequate transport and storage of the samples, freeze-thaw cycles, technical variation between laboratories, and differences between manufacturers, among others.46
Therefore, due to the general low performance of toxin EIAs, the consensus group recommends the 2-step test described in statement 9.
- 12.
The glutamate dehydrogenase assay should be the initial test when there is clinical suspicion of CDI
Quality of evidence: B1
Strength of the recommendation: strong, in favor of
Agreement reached: 100% in complete agreement
The GDH EIAs detect GDH, a constitutive enzyme that is easily detected in stool and is produced in large quantities by both toxigenic and non-toxigenic C. difficile strains, making it a good detection marker.46–49 The GDH test is the first choice in 2 and 3-step algorithms that combine it with a toxin test and/or a molecular test for detecting the toxin's gene. It is also considered a relatively simple test.46–49 It is important to state that modern techniques for GDH detection utilize monoclonal antibodies produced against the C. difficile-specific GDH, thus preventing any crossed reactivity with GDH produced by other anaerobic bacteria.49 As described in statement 9, GDH has excellent concordance, when compared with toxigenic culture, and given its high sensitivity (> 90%), it has been considered the initial test in the 2-step algorithm.50
- 13.
Colonoscopy, alone, is useful for making the differential diagnosis of other diseases (e.g., inflammatory bowel disease) or in refractory cases, but it is not recommended as an initial diagnostic test
Quality of evidence: C1
Strength of the recommendation: strong, in favor of
Agreement reached: 87% in complete agreement, 13% in partial agreement
Even though the requisites of the presence of a clinical context (> 3 unformed stools in 24h), radiographic evidence of ileus or toxic megacolon, and positive results of the abovementioned diagnostic tests have been established for the diagnosis of CDI, some authors take colonoscopic or histologic evidence of pseudomembranous colitis into account.45 However, none of the recent international guidelines mention that colonoscopy is useful in diagnosing CDI.18,47–49 The diagnostic yield of colonoscopy in the context of CDI continues to be controversial. Studies on the subject agree that, even though it is not recommended as a primary detection tool, colonoscopy can add valuable information about CDI in the presence of other colonic pathologies such as IBD, ischemic colitis, or neoplasia.73–75 Moreover, colonoscopy still has a role in the diagnosis and treatment of CDI because in more complicated cases, it enables the bowel to be decompressed and is a route for the application of medications or fecal transplantation therapy in patients with refractory CDI.76
In Mexico, there are two studies that describe endoscopic findings in CDI. Velarde-Ruiz Velasco et al.12 found endoscopic abnormalities in 87% of the patients, 38% of which were cases of pseudomembranous colitis. In their study on 306 patients, Pérez-Topete et al.77 reported colonoscopic findings in 55 of them. Thirty-five (64%) of those patients had indicative signs that were correlated with C. difficile through biopsy and culture (64%).
- 14.
Categorizing patients with confirmed CDI according to severity criteria as mild-moderate, severe, or complicated severe is recommended because it enables better therapeutic decision-making.
Quality of evidence: B1
Strength of the recommendation: strong, in favor of
Agreement reached: 100% in complete agreement
The 2010 update of the clinical practice guidelines for CDI in adults carried out by the Society for Healthcare Epidemiology of America (SHEA), the Infectious Diseases Society of America (IDSA), and the guidelines for the diagnosis, treatment, and prevention C. difficile infections published in 2013 by the American College of Gastroenterology (ACG) (Table 4) stratify the disease based on severity, which is useful for directing treatment. 26,47,48
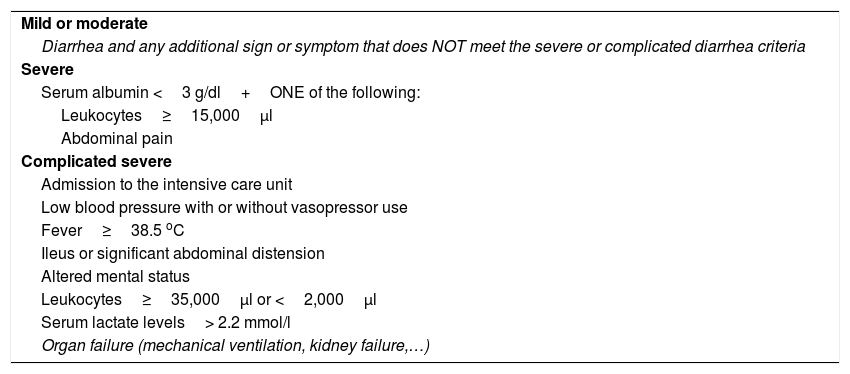
Classification of CDI severity.
| Mild or moderate |
| Diarrhea and any additional sign or symptom that does NOT meet the severe or complicated diarrhea criteria |
| Severe |
| Serum albumin <3 g/dl+ONE of the following: |
| Leukocytes≥15,000μl |
| Abdominal pain |
| Complicated severe |
| Admission to the intensive care unit |
| Low blood pressure with or without vasopressor use |
| Fever≥38.5 oC |
| Ileus or significant abdominal distension |
| Altered mental status |
| Leukocytes≥35,000μl or <2,000μl |
| Serum lactate levels> 2.2 mmol/l |
| Organ failure (mechanical ventilation, kidney failure,…) |
Adapted from Martínez-Meléndez et al.46
Finally, it should be pointed out that in the latest 2018 guidelines,48 stratification for establishing treatment changes the clinical definition of CDI only slightly, but there are no changes in the parameters, and the categories are defined as follows:
- a)
Initial non-severe episode: leukocytes <15,000/μl and serum creatinine <1.5mg/dl.
- b)
Initial severe episode: leukocytes> 15,000/μl or serum creatinine> 1.5mg/dl.
- c)
Initial fulminant episode: presence of hypotension, shock, ileus, or megacolon.
- 15.
When there is clinical confirmation of CDI, the first measure for controlling the infection and reducing the risk of progression is to suspend, whenever possible, all antimicrobial treatment, as well as all medications whose mechanisms of action increase the risk of CDI recurrence.
Quality of evidence: A1
Strength of the recommendation: strong, in favor of
Agreement reached: 83% in complete agreement, 17% in partial agreement
In a meta-analysis of 12 controlled clinical trials (CCTs)78 and a retrospective study,79 an independent association was shown between the use of antimicrobials indicated for an infection other than C. difficile and major recurrence of CDI. Therefore, the suspension of antibiotics during and after CDI is recommended, unless there is an absolute indication for continuing their use.
- 16.
Medical treatment of CDI is indicated only in the presence of symptoms. Antibiotic selection should be carried out based on severity criteria and whether the symptoms are an initial disease episode or recurrence
Quality of evidence: A1
Strength of the recommendation: strong, in favor of
Agreement reached: 83% in complete agreement, 17% in partial agreement
The treatment of CDI is indicated only in patients with diarrhea that have been diagnosed with CDI due to toxigenic C. difficile. Patients that are carriers of that microorganism should not be treated.48 For antibiotic selection, it is important to differentiate between an initial infection or a recurrent one (see statements 4 and 5). Every CDI should be stratified according to clinical symptom severity as described in statement 14.
- 17.
In the cases of initial mild-moderate Clostridium difficile infection, oral vancomycin at a dose of 125mg every 6h is recommended as the treatment of choice. If vancomycin is unavailable, metronidazole is the recommended alternative treatment
Quality of evidence: A1
Strength of the recommendation: strong, in favor of
Agreement reached: 96% in complete agreement, 4% in partial agreement
Oral metronidazole and vancomycin are the most widely used antibiotics in the treatment of mild-moderate CDI. Two CCTs showed that the efficacy of metronidazole was similar to that of vancomycin.80,81 In a recent study, efficacy for curing moderate infection was reported at 90% for metronidazole and 98% for vancomycin.7 In another CCT,82 vancomycin was found to be superior to metronidazole in cases of severe infection. We recommend the use of metronidazole as alternative treatment for mild-moderate CDI if vancomycin is not readily accessible. Metronidazole has the advantage of being considerably less expensive than vancomycin and is widely available in Mexico. It is prescribed at a dose of 500mg, three times a day, for 10 days. In patients that do not tolerate the medication orally, it can be administered intravenously.47 Lack of response to metronidazole after 5 days of treatment is an indication for changing the antibiotic to oral vancomycin at a dose of 125mg every 6h for 10 days.47,48
- 18.
In cases of initial severe CDI, treatment should always be oral vancomycin for 14 days
Quality of evidence: B1
Strength of the recommendation: strong, in favor of
Agreement reached: 100% in complete agreement
Oral vancomycin is the antibiotic of choice for severe CDI. Several CCTs have shown its superiority to metronidazole.7,81,82 A dose of 125 to 250mg every 6h for 10 to 14 days should be prescribed, depending on the clinical response of the patient. In Mexico, there is no oral presentation of the drug. It is only available in vials for intravenous administration. The oral use of the IV presentation has been shown to be effective in the treatment of CDI, due to the fact that it is barely absorbed and thus reaches high concentrations in the colon. The recommendation is to add 10ml of sterile injectable water to the 500mg vial and give 2.5ml (125mg) to the patient every 6h in fruit juice, such as apple or pear, for better tolerance. A recent pre-clinical study conducted in Mexico showed that the bioavailability of vancomycin administered orally in injectable water was more stable over time and reached higher concentrations in the colon than when administered in saline solution and orange juice.83
- 19.
In cases of complicated severe CDI, treatment should be oral vancomycin combined with intravenous metronidazole
Quality of evidence: A1
Strength of the recommendation: strong, in favor of
Agreement reached: 87% in complete agreement, 13% in partial agreement
The combination treatment of oral vancomycin at 250 to 500mg every 6h and intravenous metronidazole at 500mg every 8h is the treatment of choice for complicated severe CDI.26,47 There are no clinical trials that enable the regimen of antibiotic doses in complicated severe CDI to be evaluated and the therapeutic regimen described herein is based on the recommendations of experts.26,47,48 However, in a recent clinical study, the authors observed that the addition of intravenous metronidazole to vancomycin decreased patient mortality by 50%.84
In cases of complicated severe CDI that present with great abdominal distension or ileus, 500mg of vancomycin through a rectal tube every 6h is recommended.26
- 20.
Other antimicrobial treatment alternatives for CDI are: fidaxomicin, rifaximin-alpha, and nitazoxanide
Quality of evidence: B1 for fidaxomicin, C1 for rifaximin and nitazoxanide
Strength of the recommendation: strong, in favor of
Agreement reached: 78% in complete agreement, 18% in partial agreement, 4% uncertain
Treatment with fidaxomicin at a dose of 200mg, bid, for 10 days, is an alternative to vancomycin. Recent clinical trials have shown the non-inferiority of fidaxomicin, compared with vancomycin. It has even been suggested in a post hoc analysis that fidaxomicin has a lower recurrence rate than vancomycin.85,86 Fidaxomicin is not yet available in Mexico.
The polymorph alpha form of rifaximin is a non-absorbable antimicrobial that reaches high concentrations in stool. Its efficacy and safety have been studied at a dose of 400mg every 8h for 20 days, alone, or combined with first-line therapy and in cases of recurrent CDI, with modest results, and the evidence suggests that it can be used as a coadjuvant in regimens that include vancomycin for the treatment of recurrent CDI.18,48,87 Nitazoxanide has been compared with metronidazole and vancomycin treatment regimens. The sustained response of the three antibiotics at 31 days was similar.88 A larger number of well-designed studies are needed to determine the efficacy of that antibiotic.
- 21.
For the treatment of the first recurrence of CDI, vancomycin is recommended if the patient received metronidazole. If vancomycin was prescribed, retreatment with vancomycin is recommended, followed by tapering doses, and ending with pulsed doses
Quality of evidence: C2
Strength of the recommendation: weak, in favor of
Agreement reached: 100% in complete agreement
The recurrence of CDI after an initial episode is 10-20%, but when a patient presents with a first recurrence, the possibility of future infection relapses increases to 40 and to 65%, as described in statement 5.47,89
For the treatment of the first recurrence of CDI, oral vancomycin is recommended, if the initial antibiotic was metronidazole. If initial treatment was vancomycin, then the same treatment is recommended, followed by a regimen of tapering doses, ending with pulsed doses, under the following regimen: 125mg every 12h for one week, followed by 125mg once a day for one week, and then 125mg every 2 or 3 days, for two to eight weeks.
- 22.
When there is a second recurrence, oral vancomycin for 14 days is recommended, with tapering doses and finally pulsed doses
Quality of evidence: C2
Strength of the recommendation: weak, in favor of
Agreement reached: 96% in complete agreement, 4% in partial agreement
In CDI episodes after the first relapse, vancomycin at a dose of 125mg every 6h for 10 to 14 days is recommended, followed by tapering doses of vancomycin, ending with pulsed doses, under the same regimen presented in the previous statement, despite the fact that there are no controlled clinical trials to support that type of regimen.
Other alternatives are 125mg of vancomycin every 6h for 10 to 14 days, followed by 400mg of rifaximin every 8h for 20 days or 200mg of fidaxomicin every 12h for 10 days, if it becomes available in Mexico in the future.
- 23.
The evidence for recommending probiotics in the prevention of CDI or its recurrence is limited to certain strains
Quality of evidence: B1
Strength of the recommendation: strong, in favor of
Agreement reached: 100% in complete agreement
There are very few studies evaluating the usefulness of probiotics in the treatment of initial CDI and therefore data are insufficient for supporting the use of probiotics as adjuvants in the treatment of an active infection with antibiotics.47,48
On the other hand, different meta-analyses have evaluated the usefulness of probiotics in the prevention of antibiotic-associated diarrhea caused by C. difficile (AADCD). A Cochrane review90 recently evaluated 39 studies on 9,955 participants, including adults and children. In 31 of the studies, the results suggested that probiotics reduced the risk for AADCD by 60%. The incidence of AADCD was 1% in the patients treated with probiotics and 4% in those that received placebo. In the post hoc analysis, the authors showed that the reduction in AADCD was significantly higher with probiotics than with placebo (3.1 versus 11.6%; NNT=12) in the population with a baseline risk> 5% for developing CDI. That effect was not observed in the subjects with a baseline risk <5% for presenting with CDI.
Different CCTs, systematic reviews, and management guidelines have published controversial results with respect to the use of probiotics in the prevention of CDI in adult hospitalized patients that receive antibiotics.91–93 In a recent meta-analysis, 19 studies on 6,261 adults admitted to the hospital under treatment with antibiotics were evaluated. It showed that the incidence of CDI in the cohort that received probiotics was 1.6%, which was significantly lower than the incidence in controls of 3.9% (p <0.001).91 The accumulated relative risk in the patients treated with probiotics was 0.42 (95% CI: 0.30-0.57). The meta-regression analysis showed that probiotics were significantly more effective if administered close to the first dose of antibiotics.93 Probiotics administered within the first two days of antibiotic therapy commencement produced a greater reduction in the risk for CDI (relative risk: 0.32; 95% CI: 0.22-0.48) than late administration (relative risk: 0.70; 95% CI: 0.40-1.23). A high number of adverse events was not demonstrated with probiotics. That systematic review with a meta-analysis showed that probiotics administered close to the first dose of antibiotics reduced the risk for CDI in> 50% of adult hospitalized patients treated with antibiotics.93
The most widely studied strains of probiotics that have shown benefits in the prevention of CDI recurrence are Lactobacillus acidophilus CL1285+L. casei LBC8OR; Saccharomyces boulardii CNCM I-745; Lactobacillus rhamnosus HN002+Lactobacillus acidophilus NCFM; and Lactobacillus acidophilus+Bifidobacterium bifidum (Cultech strains). 90–93
- 24.
Fecal microbiota transplantation is a safe and effective option in patients with CDI with two recurrences or in patients with severe episodes and antimicrobial treatment failure
Quality of evidence: B1
Strength of the recommendation: strong, in favor of
Agreement reached: 100% in complete agreement
Fecal microbiota transplantation (FMT) consists of the infusion of stool (that contains the complete gut microbiota community) from a healthy donor into the digestive tract of a patient to cure or improve a disease. The aim of FMT in CDI is to restore the diversity of microorganisms in the colonic microbiota and eliminate the growth of C. difficile. Case series, CCTs, and different meta-analyses have shown that FMT is effective in curing recurrences in 85 to 90% of the cases treated.94,95 The indications for FMT are:
- •
Recurrent Clostridium difficile infection
- ∘
At least 3 episodes of mild or moderate CDI (one initial and 2 recurrent) with failure of treatment with vancomycin for 6 to 8 weeks, with or without an alternate antibiotic (fidaxomicin, rifaximin, nitazoxanide).
- ∘
At least 2 episodes of CDI with hospital admission and significant morbidity.
- •
Severe or fulminant CDI that does not respond to standard treatment within 48h.
Donor selection is a very important step for the safety and success of FMT. Studies have shown that fecal microbiota from a known donor (relative, friend, spouse) or a universal donor (anonymous) can be used. The donor should be healthy with no digestive or extradigestive comorbidities and no risk for transmitting an infectious agent, as well as not having taken an antibiotic within the past 3 months. Donor selection strategy has been described in several articles.96,97 FMT administration routes include a nasogastric tube, colonoscopy, enemas, or capsules, and all have been shown to be effective.98 Likewise, FMT efficacy is similar using fresh or frozen fecal microbiota.99,100
In patients with severe CDI, FMT has also been shown to be effective in more than 80% of the cases treated. The detection of pseudomembranous colitis during CDI is an indication for sequential FMT, with or without vancomycin administration, until achieving clinical remission.100,101 Even though evidence is limited, FMT has also been shown to be effective in immunocompromised patients with CDI.102
The short-term adverse effects are minor and include transitory fever, malaise, or abdominal pain. Norovirus and rotavirus transmission, as well as cases of death associated with the infusion route of FMT, such as bronchoaspiration with nasogastric tube administration or perforation associated with colonoscopy, have been described. In general, FMT is accepted to be a safe procedure. The long-term effects are still unknown, but there is the potential risk for developing allergies, autoimmune diseases, inflammatory bowel disease, obesity, and other metabolic disorders, or the transmission of a disease of the donor. Therefore, treatment with FMT should only be used in patients with CDI with the abovementioned indications.48,99–102
- 25.
Surgery should be reserved for patients with severe colitis (toxic megacolon) that have had failure with all medical treatment, failed FMT, generalized peritonitis, or the rare cases of colonic perforation. The procedure of choice is subtotal colectomy with ileostomy
Quality of evidence: B1
Strength of the recommendation: strong, in favor of
Agreement reached: 91% in complete agreement, 9% in partial agreement
Surgical treatment is indicated in patients that develop low blood pressure and require vasoconstricting amines, clinical signs of sepsis, or multiple organ failure with clinical evidence of toxic megacolon, peritonitis, or perforation. Surgery should also be considered in patients with severe CDI that do not respond to treatment with antibiotics or with FMT. The surgical procedure of choice is subtotal colectomy with end ileostomy. Segmented colectomies have a worse prognosis than subtotal colectomy.12 Other therapeutic modalities include loop ileostomy with intraoperative lavage with polyethylene glycol and postoperative anterograde infusion of vancomycin. In a randomized trial, that procedure contributed to the preservation of the colon in 90% of the cases and to a lower surgical mortality rate, compared with historic controls (19 vs 50%).103
- 26.
There are measures of prevention and control of CDI directed at the interruption of the bacterium's mechanism of transmission through the hands of patients and healthcare professionals and the handling of surfaces or medical devices contaminated by the bacterial spores
Quality of evidence: B1
Strength of the recommendation: strong, in favor of
Agreement reached: 100% in complete agreement
Hand hygiene is the cornerstone of nosocomial infection prevention. Appropriate hand-washing with soap and water, not alcohol, before and after seeing patients with CDI is effective for removing C. difficile spores on the medical personnel. The isolation of patients with CDI in a room with a private bathroom is recommendable and should be continued for 48h after the diarrhea is resolved. The personnel in contact with patients with CDI should wear gloves and a medical gown when seeing them. The instruments or diagnostic devices used on patients with CDI should be disinfected with sporicides and rooms should be cleaned and disinfected with sporicidal agents in cases of a hospital outbreak of CDI.48
- 27.
The implementation of optimization and education programs on antibiotic use (antimicrobial stewardship), as well as in-hospital epidemiologic controls, environmental decontamination, and hand-washing are measures that have demonstrated a decrease in the incidence of CDI
Quality of evidence: B1
Strength of the recommendation: strong, in favor of
Agreement reached: 100% in complete agreement
The reduction in the frequency of use, duration, and number of high-risk antibiotics in the hospitalized patient, reduces the risk for CDI. Hospital antibiotic use should be based on local epidemiology and patient culture results. The restriction of fluoroquinolones, clindamycin, and cephalosporins should be considered a very important measure in the prevention of and solution to the problem in medical institutions. In addition, the implementation of antimicrobial stewardship programs is obligatory, especially in tertiary care hospital centers.48
ConclusionsThe present document contains 27 statements that provide recommendations for the prevention, diagnosis, and treatment of Clostridium difficile infection, directed at physicians that treat adults. CDI should be suspected in subjects with diarrhea that have a history of antibiotic use and/or immunosuppression, but the infection can also be community-acquired. The implementation of a 2-step diagnostic algorithm is proposed. It first utilizes a high sensitivity test, such as the glutamate hydrogenase (GDH) assay, which if positive, should be confirmed by toxin detection through the immunoassay technique or nucleic acid amplification tests. Categorizing CDI based on clinical evaluation into mild-moderate, severe, and complicated severe is recommended, because it enables better therapeutic decision-making. Oral vancomycin is the medication of choice in mild-moderate CDI, but metronidazole can be an alternative. Fecal microbiota transplantation is recognized as an efficacious option in very severe cases or when the infection is recurrent. Surgery should be reserved for patients with severe colitis (toxic megacolon) in whom all medical treatment has failed.
Financial disclosureThe present consensus was carried out with the support of the Asociación Mexicana de Gastroenterología, which enabled the participation, transport, and accommodations during the face-to-face voting. No fees were received.
Conflict of interestDr. Ana Teresa Abreu y Abreu: is or has been a Speaker for Takeda, Sanofi, Mayoli-Spindler, Alfa-Wassermann, and Carnot.
Dr. José Antonio Velarde-Ruiz Velasco: is or has been a Speaker for Takeda, Asofarma, Alfa-Wassermann, Abbvie, Abbott, Gilead, and MSD.
Dr. Mónica Rocío Zavala-Solares: is or has been a Speaker for Takeda.
Dr. José María Remes-Troche: is a Member of the Advisory Board of Takeda Pharmaceuticals, Alfa-Wassermann, and Almirall. He has received research funds from Sanfer. He is a Speaker for Takeda, Asofarma, Alfa-Wassermann, Carnot, Almirall, and Astra-Zeneca.
Dr. Ramón Isaías Carmona-Sánchez: is or has been a Speaker for Asofarma, Astra-Zeneca, and Chinoin.
Dr. Juan Manuel Aldana Ledesma: declares he has no conflict of interest.
Dr. Adrián Camacho-Ortiz: declares he has no conflict of interest.
Dr. Raúl Contreras Omaña: declares he has no conflict of interest.
Dr. Raymundo Díaz Seoane: declares he has no conflict of interest.
Dr. Carolina Tatiana Elizondo Vázquez: declares she has no conflict of interest.
Dr. Elvira Garza-González: declares she has no conflict of interest.
Dr. Octavio Gómez-Escudero: is or has been a Speaker for Takeda.
Dr. Janett Sofía Jacobo Karam: declares she has no conflict of interest.
Dr. Sergio A. Lazo de la Vega Jasso: declares he has no conflict of interest.
Dr. Miguel Morales Arámbula: is or has been a Speaker for Takeda and Asofarma.
Dr. Laura Ofelia Olivares Guzmán: declares she has no conflict of interest.
Dr. José Sifuentes Osornio: declares he has no conflict of interest.
Dr. Ana Guadalupe Siu Moguel: declares she has no conflict of interest.
Dr. Rodrigo Soto Solís: is or has been a Speaker for Ferring, Novartis, and UCB.
Dr. Luis Raúl Valdovinos García: declares he has no conflict of interest.
Dr. Miguel Ángel Valdovinos Díaz: is a Member of the Advisory Board of Takeda, Menarini, Sanofi, Biocodex. He is a Speaker for Takeda, Sanofi, Menarini, Biocodex, and Carnot.
Dr. Genaro Vázquez Elizondo: is or has been a Speaker for Takeda, Asofarma, and Sanfer.
Dr. G. Grajales Figueroa: declares he has no conflict of interest.
Please cite this article as: Abreu y Abreu AT, Velarde-Ruiz Velasco JA, Zavala-Solares MR, Remes-Troche JM, Carmona-Sánchez RI, Aldana-Ledesma JM, et al. Consenso sobre prevención, diagnóstico y tratamiento de la infección por Clostridium difficile. Revista de Gastroenterología de México. 2019;84:204–219.