Cow's milk protein allergy (CMPA) is the most frequent cause of food allergy in the first months of life. Despite the fact that there are different guidelines and recommendations on the management of children with CMPA, there continues to be great variability in diagnostic and therapeutic criteria in Latin America. The Food Allergy Working Group of the Latin American Society for Pediatric Gastroenterology, Hepatology and Nutrition summoned a group of Latin American experts to reach a consensus and formulate a document to unify diagnostic and therapeutic criteria for CMPA. Three teams were formed, each with a coordinator, and the members of each team developed a series of statements for their corresponding module: a) clinical manifestations and diagnosis; b) diagnostic tools, and c) treatment. A search of the medical literature was carried out to support the information presented in each module and 28 statements were then selected. The statements were discussed, after which they were evaluated by all the experts, utilizing the Delphi method. Their opinions on statement agreement or disagreement were anonymously issued. The final statements selected were those with above 75% agreement and their corresponding recommendations were formulated, resulting in the document presented herein.
La alergia a las proteínas de leche de vaca (APLV) constituye la causa más frecuente de alergia alimentaria en los primeros meses de vida. A pesar de la existencia de diferentes guías y recomendaciones sobre el manejo de niños con APLV, en Latinoamérica sigue observándose una gran variabilidad de criterios diagnósticos y terapéuticos. El grupo de trabajo de Alergia Alimentaria de la Sociedad Latinoamericana de Gastroenterología, Hepatología y Nutrición Pediátrica se dio a la tarea de convocar a un grupo de expertos de la región, realizar un consenso y elaborar un documento con el objetivo de unificar criterios diagnósticos y terapéuticos para APLV. Se dividió el grupo en tres equipos bajo un coordinador para cada equipo, y los miembros de cada grupo formularon una serie de enunciados correspondientes a uno de tres módulos diferentes: a) manifestaciones clínicas; b) herramientas diagnósticas, y c) tratamiento. Se buscó la información en la literatura médica para sustentar la información de cada uno de ellos, y posteriormente se seleccionaron 28 enunciados, los cuales fueron discutidos y posteriormente evaluados por todos los expertos, a través de método Delphi, quienes emitieron su opinión sobre acuerdo o desacuerdo sobre las mismas de forma anónima. Todos los enunciados obtuvieron porcentajes de acuerdo mayores al 75%, por lo que permanecieron, y con base en ellos se elaboraron las recomendaciones y se presentan.
Food allergies are a health problem that has been increasing in recent years. They include a broad spectrum of disorders that result from adverse immune responses to food antigens. Cow’s milk proteins (CMPs), some of the first non-human dietary proteins ingested by children, are the most frequent cause of food allergies in the first months of life. Cow’s milk protein allergy (CMPA) is the result of an abnormal immune response that occurs after the ingestion of CMPs and its mechanisms are immunoglobulin E (IgE)-mediated, non-IgE-mediated, or mixed. According to a 2014 meta-analysis, the prevalence of CMPA, verified by the oral food challenge (OFC), was 0.6% (0.5-0.8 %).1 Incidence in the first year of life is estimated at 2 to 3%.2 There are no studies showing the overall prevalence of CMPA in Latin America. Studies from Brazil report an incidence of 2.2% and a prevalence of 5.4%.3 In Argentina, a study conducted at a community university hospital reported a prevalence of 0.88% in children with CMPA, diagnosed through OFC.4 In a 2014 study carried out in Chile, a 4.9% incidence of CMPA was identified in children under one year of age.5 Distinct factors can explain the differences in prevalence, and how the diagnosis is made and corroborated is a primary one. Even though there are guidelines and recommendations on the management of children with CMPA, there continues to be great variability of diagnostic and therapeutic criteria in Latin America. The Food Allergy Working Group of the Latin American Society for Pediatric Gastroenterology, Hepatology and Nutrition (LASPGHAN) recently published the results of a survey that showed much diversity in the diagnostic and therapeutic approach, as well as poor adherence to the existing guidelines.6 In Brazil, similar findings were described in previous studies, showing an adherence of 16.7% to the national and international guidelines on CMPA.7,8 Thus, the Food Allergy Working Group of the LASPGHAN decided to summon a group of Latin American experts to search for the best scientific evidence and carry out a consensus for the region, formulating a document to unify diagnostic and therapeutic criteria for CMPA in Latin America. The document presented herein is the result of that process and is directed at pediatric gastroenterologists, pediatric nutriologists, pediatric nutritionists, as well as at primary pediatricians and healthcare professionals that can opportunely detect the disease and refer the patient to the specialist.
MethodThe coordinators of the Food Allergy Working Group of the LASPGHAN (MCT, RVF, MBM) were responsible for the development of the guidelines and summoned a group of Latin American specialists to participate. They formulated pertinent questions, selecting the topics of greater relevance, according to the controversies identified in the previous survey.6 The members of the working group were divided into 3 teams, each with a coordinator, encompassing the themes of 1) clinical manifestations and diagnosis, 2) diagnostic tools, and 3) treatment. Search protocols for gathering the evidence were established through validated strategies and the MeSH terms utilized were: allergy, food allergy, CMPA, clinical manifestations, diagnostic test, diagnostic algorithms, elimination test, oral food challenge, challenge test, specific IgE test, patch test, extensively hydrolyzed cow’s milk proteins, extensively hydrolyzed serum, extensively hydrolyzed casein, elemental formula, amino acid formula, soy formula, tolerance acquisition, complementary feeding, milk protein, supplements. The database searches included articles in Spanish, English, and Portuguese; studies on humans (children and adolescents 0-18 years of age), clinical practice guidelines, meta-analyses, randomized placebo-controlled trials, consensuses, reviews, and official regulations. Statements corresponding to each of the themes to be covered were formulated in response to the questions and discussed within each group, and then among all the participants. Afterwards, all the experts anonymously gave their opinions, regarding their agreement or disagreement with the statements. This was done virtually, utilizing an online questionnaire, to reduce opinion dispersion and specify the average of the consensus opinions. When 75% agreement was obtained, the recommendations on diagnosis and treatment were formulated. The statements below 75% agreement were reviewed, re-analyzed, and voted upon again, or eliminated. Given the nature of the document, no ethics considerations were involved, nor was approval by an ethics committee required.
ResultsModule 1. Clinical manifestations and diagnosisThe clinical manifestations in infants with CMPA may depend on the immune mechanism involved: IgE-mediated, non-IgE-mediated, or a mixed mechanism. Reactions dependent on the time they occur from ingestion to symptom onset, are described as immediate, when they appear within minutes or up to 2 h after allergen ingestion, and as delayed, when they appear from 48 h to a few weeks after ingestion.9 Their intensity is varied, ranging from very mild to very severe.9,10 The most frequent symptoms for the diagnostic suspicion of CMPA, especially in infants, are: irritability, intense crying, cutaneous reactions, such as atopic dermatitis, urticaria, and angioedema, and gastrointestinal symptoms, such as vomiting or regurgitation, abdominal pain, persistent abdominal cramping with pathologic characteristics, constipation, or diarrhea, with or without intestinal bleeding, the majority of which present before 12 months of age.11
Statement 1. Symptoms of IgE-mediated CMPA are diverse and involve a variety of organs and systems. The skin and mucosa are more frequently affected, whereas gastrointestinal and respiratory tract symptoms are less common.
Agreement percentage: 97.22%
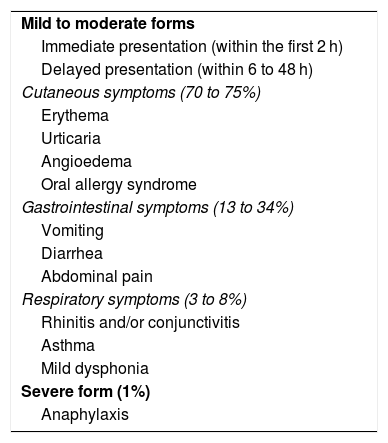
IgE-mediated reactions are characterized by immediate onset, within the first 2 h after CMP ingestion, of predominantly cutaneous symptomatology. Later reactions that appear between 6 and 48 h after CMP ingestion, with lower frequency, are also described (Table 1). Clinical manifestations are predominantly cutaneous (70-75%), and less frequently, gastrointestinal (13-34%) and respiratory (1-8%); the most severe form is anaphylaxis, which can present from 1 to 4%.12–14 The possibility of more than one compromised organ or system is described in 26% of patients.15–17
Clinical manifestations of immunoglobulin E-mediated cow’s milk protein allergy.
| Mild to moderate forms |
| Immediate presentation (within the first 2 h) |
| Delayed presentation (within 6 to 48 h) |
| Cutaneous symptoms (70 to 75%) |
| Erythema |
| Urticaria |
| Angioedema |
| Oral allergy syndrome |
| Gastrointestinal symptoms (13 to 34%) |
| Vomiting |
| Diarrhea |
| Abdominal pain |
| Respiratory symptoms (3 to 8%) |
| Rhinitis and/or conjunctivitis |
| Asthma |
| Mild dysphonia |
| Severe form (1%) |
| Anaphylaxis |
In the diagnostic algorithm, the clinical history is a fundamental step, with a complete medical interview that includes a characterization of the allergic reaction-inducing foods, symptoms (when they occur, sequence, duration, frequency, repetition, reproducibility), time from food intake to symptom onset, circumstances right before symptom onset, age at onset, dietary details (formula-fed or breastfed), history of other eliminated foods and treatment, comorbidities (gastroenteritis or other infections), appetite, growth curves, and family history of allergy.18–20
There can be three different clinical reactions in children, with respect to cutaneous symptoms: immediate-type reactions, isolated late eczematous reactions, and combined immediate-type and late eczematous reactions.14 The immediate-type IgE reactions present with erythema, urticaria, or angioedema in more than 50% of patients. Ten to 15% present only with perioral erythema. Temporary episodes are called acute, whereas episodes that are repeated for more than 6 weeks are called chronic. Urticaria is the clinical presentation that is seen most frequently.21–24 CMP contact urticaria occurs only in patients with IgE-mediated CMPA and is more frequently associated with atopic dermatitis.25 Angioedema is the second most frequent dermatologic presentation; it is asymmetrically distributed and involves non-gravitational areas.24,25 It is located on the face, the extremities, or the upper airway. Pruritus is less frequent.16 Oral allergy syndrome is the inflammation of the lips, tongue, and cheeks after contact with food in the oral cavity. Pruritus or a tingling sensation and angioedema of the lips are immediate reactions.16 Isolated respiratory symptoms, such as acute rhino-conjunctivitis with watery rhinorrhea, sneezing, tearing of the eyes, asthma, or episodes of mild, self-limited dysphonia are rare, as single manifestations of IgE-mediated CMPA.15,16 Gastrointestinal symptoms are also an infrequent clinical manifestation in IgE-mediated CMPA. It presents as immediate gastrointestinal hypersensitivity, with symptoms that can include nausea, vomiting, acute abdominal pain, and sudden-onset diarrhea, after CMP ingestion. Affected infants can present with vomiting, food rejection, crying, and irritability.15,16 Finally, anaphylaxis is a severe IgE-mediated reaction that appears immediately after CMP intake, is multisystemic, rapid, and progressive.26–29
Statement 2. CMPA in infants that is caused by mixed mechanisms (IgE-mediated and non-IgE-mediated) presents with skin-related symptoms: atopic dermatitis, and more rarely, eosinophilic esophagitis.
Agreement percentage: 95.83%
The clinical manifestations of CMPA that is mediated by a mixed mechanism, in infants, are AD and eosinophilic esophagitis; the former is the most frequent and the latter is much less frequent.30 Atopic dermatitis is a chronic and recurrent inflammatory disease of the skin, characterized by dry skin and a low threshold to pruritus, that is often associated with allergic sensitization, an increase in serum IgE, or with the mixed component.31 It is one of the manifestations of CMPA that causes more diagnostic doubt, given the difficulty of relating lesion worsening to CMP intake.22 To make its diagnosis, it is important to keep in mind that it is more frequent in infants and children and that it appears on the face, neck, and extremities, and that it has certain presentation characteristics determined by age at onset, severity, and allergen. A localized presentation of atopic dermatitis associated with CMPA is umbilical and periumbilical erythema (red umbilicus). CMP has been established as a possible causal allergen in approximately one-third of children under 12 months of age, with moderate-to-severe early onset AD (first trimester of life).19,22,31,32 For the diagnosis of AD and the evaluation of its severity, the affected surface, pruritus, sleep disorders, the impact on daily activities, and disease persistence must be kept in mind.33–36
Statement 3. Non-IgE-mediated CMPA clinically presents with the delayed onset of predominantly digestive symptoms, including three syndromes: proctocolitis, enteropathy, and CMP-induced food protein-induced enterocolitis syndrome (FPIES); or with symptoms resembling functional gastrointestinal disorders: infant colic, infant regurgitation, and constipation.
Agreement percentage: 100%
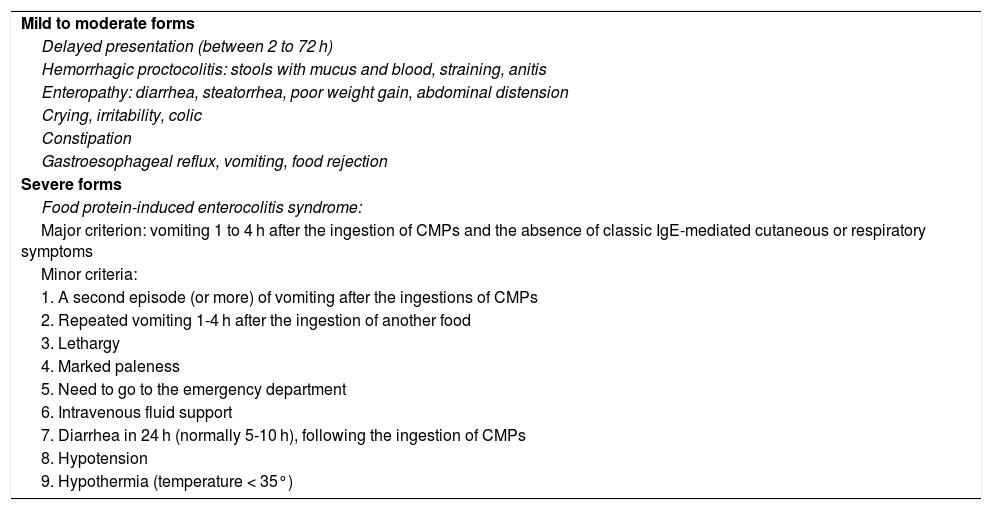
The non-IgE-mediated forms are generally due to cellular immune responses, albeit the immune mechanism is not identified (Table 2).37 Gastrointestinal symptoms are variable and prominent, affecting the entire gastrointestinal tract.38,39 Clinical manifestations are varied and can present as vomiting, regurgitation, discomfort, hyporexia, anorexia, or food rejection. Bowel movement alterations are frequent, with diarrhea that is due to enteropathy, which can cause delayed growth, or that is due to colitis, with straining and/or blood and mucus in the stools. Constipation associated with perianal lesions can also be present, and colicky abdominal pain is not uncommon. Chronic iron-deficiency anemia can be the sole manifestation of CMPA in infants and children. Growth failure is a nonspecific presentation and can have severe nutritional consequences.12,37–44 Late-onset symptomatology, predominantly digestive, includes three syndromes: proctocolitis, enteropathy, and FPIES.
Clinical manifestations of non-immunoglobulin E-mediated cow’s milk protein allergy.
| Mild to moderate forms |
| Delayed presentation (between 2 to 72 h) |
| Hemorrhagic proctocolitis: stools with mucus and blood, straining, anitis |
| Enteropathy: diarrhea, steatorrhea, poor weight gain, abdominal distension |
| Crying, irritability, colic |
| Constipation |
| Gastroesophageal reflux, vomiting, food rejection |
| Severe forms |
| Food protein-induced enterocolitis syndrome: |
| Major criterion: vomiting 1 to 4 h after the ingestion of CMPs and the absence of classic IgE-mediated cutaneous or respiratory symptoms |
| Minor criteria: |
| 1. A second episode (or more) of vomiting after the ingestions of CMPs |
| 2. Repeated vomiting 1-4 h after the ingestion of another food |
| 3. Lethargy |
| 4. Marked paleness |
| 5. Need to go to the emergency department |
| 6. Intravenous fluid support |
| 7. Diarrhea in 24 h (normally 5-10 h), following the ingestion of CMPs |
| 8. Hypotension |
| 9. Hypothermia (temperature < 35°) |
CMPs: cow’s milk proteins.
Proctitis, or proctocolitis, predominantly presents between 2 and 8 weeks of age, and less frequently up to 6 months of age, in infants that are exclusively breastfed or receive CMP formula. Generally, those children are in good overall condition, with good weight and height development. Bright red blood in stools, appearing as dots, streaks, or small clots, usually with mucus, is the main characteristic of the condition. Infants tend to present with colic, crying and irritability, gases, abdominal pain, vomiting, and discomfort during bowel movements.37,42,43 Hematochezia in neonates could be associated with neonatal transient eosinophilic colitis, which must be differentiated from CMPA.45,46 Enteropathy can begin within the first 9 months and is more frequent in infants under 6 months of age. It is a rare syndrome with small bowel lesions, causing intestinal malabsorption similar to celiac disease, but with less severe lesions, and has become less frequent over the past decade.44 Infants present with diarrhea (with or without vomiting), abdominal distension, and more than 50% have growth failure, especially when they also present with chronic diarrhea. Symptoms can begin gradually or suddenly and can be prolonged and worsen, with intestinal malabsorption, steatorrhea, and weight loss; even anemia and hypoalbuminemia can present, behaving as a protein-losing enteropathy.37,42–44,47 FPIES is a severe form of non-IgE-mediated CMPA that presents a few hours after allergen ingestion, and requires emergency treatment, with intravenous fluid and electrolyte replacement.37,48 The involved food varies in different regions of the world. In the United States, the most frequent is CMP, together with soy, rice, and oatmeal. Reactions to different grains, fruits, vegetables, and meats have also been seen.49 The clinical manifestations and severity of FPIES depend on the frequency and dose of the involved food, age of the patient, and association with another IgE-mediated allergy.48,50 The possibility of the association between FPIES and other atopic conditions, such as AD and other IgE-mediated food allergies, is important to keep in mind.48,51 With respect to FPIES, the clinical history is fundamental and sufficient for making the diagnosis and identifying the culprit food.37,50,52–56 Given the severity of the symptoms and the impossibility of performing a provocation test to confirm the diagnosis, the diagnostic criteria for the acute and chronic forms were established by consensus. In the acute form, vomiting begins within 1 to 4 h after intake, and occurs when ingestion is intermittent or there was a period of suspension. It is accompanied by watery diarrhea, rarely with mucus and blood, that appears between 5 and 10 h and can last for 24 h. Symptoms resolve 24 h after ingestion. Between episodes, infants are asymptomatic and have a normal growth curve.50 Diarrhea with blood is significantly more likely in infants under 2 months of age that present with FPIES due to CMPA, compared with infants that have symptoms after 2 months of age.50 The chronic form of FPIES is less characterized than the acute form and is only described in infants under 4 months of age that receive CMP formula. It develops in children with regular and repeated CMP formula ingestion that present with chronic and intermittent vomiting, watery diarrhea, nutritional disorders, hypoalbuminemia, and poor weight gain, and can also cause dehydration and shock.50 FPIES is often misdiagnosed as viral gastroenteritis, food intoxication, sepsis, or a surgical disease.52,53
The clinical manifestation of CMPA can also resemble functional gastrointestinal disorders, such as infant colic, infant regurgitation, and constipation.57–66 CMPA is considered rare in infants with colic as the sole symptom.58 Infant colic and AD in combination, abnormal bowel movements, blood in stools, infant regurgitation, or cough and bronchial spasm merit the elimination of CMP to rule out CMPA.58,62–64 Discomfort and crying can be associated with infant regurgitation and/or CMPA.57 The relation between infant regurgitation and gastroesophageal reflux disease (GERD) associated with CMPA is not yet clear, and distinguishing between primary GERD and GERD that is secondary to a food allergy is difficult. The diagnosis of CMPA should be considered when there is gastroesophageal reflux, irritability, and particularly, if they are associated with other atopic symptoms, such as eczema.58–62 Guidelines on constipation recommend considering CMPA as a possible cause in infants. In those cases, it is usually associated with few and delayed bowel movements, soft stools, prolonged excess straining, and with a normal or slightly distended abdomen. Constipation is more common in children with CMPA that are formula-fed than in those that are exclusively breastfed.58,62,63 Growth failure is another symptom that should be considered a clinical manifestation of CMPA, especially in the non-IgE-mediated forms.67
Statement 4. The most frequent clinical presentation of CMPA in exclusively breastfed children is non-IgE-mediated proctocolitis, with little or no general involvement, that has good recovery after CMP is suspended from the diet of the mother and from the diet of the infant, at one year of age.
Agreement percentage: 100%
CMP is the allergen most frequently involved in food allergy, in exclusively breastfed children. CMP is detected in the breast milk of mothers that drink cow’s milk, as beta-lactoglobulin, at levels from 0.9 to 150 µg/l. Soy, eggs, and wheat are other allergens capable of inducing symptoms, but much less frequently. The most frequent clinical manifestation of CMPA is gastrointestinal, and the presentation is mild hemorrhagic proctocolitis. It rarely presents as enteropathy or IgE-mediated CMPA. There are few cases described worldwide of FPIES due to CMP, in exclusively breastfed infants under 6 months of age. There is little evidence for suspecting CMPA in exclusively breastfed children that present with isolated symptoms of vomiting or regurgitation, colic, or constipation.59
Module 2. Diagnostic toolsStatement 5. The diagnosis of CMPA is clinical and requires no laboratory tests for its corroboration.
Agreement percentage: 93%
The diagnosis of CMPA is essentially clinical and requires no laboratory tests for its corroboration. Avoiding ingestion of the allergen is the diagnostic test utilized to confirm CMPA, which is then followed by an oral challenge.38 As previously described herein, a detailed clinical history can facilitate the diagnostic approach. In recent years, with the knowledge that there is no biomarker that can be used for diagnosing CMPA, especially the non-IgE-mediated forms, the Cow’s Milk-related Symptom Score (CoMiSS) has been developed for use in cases of suspected CMPA35,68–72 Other tools that have been used are allergen-specific IgE quantification or the support of a pediatric allergist that can aid in carrying out the skin prick test (SPT) and patch test.73 None of those tools, alone, can diagnose CMPA.
Statement 6. The open oral food challenge is useful for confirming the diagnosis of CMPA and characterizing the development of oral tolerance, especially in infants.
Agreement percentage: 95.83%
Statement 7. Some patients, generally older and with IgE-mediated reactions, may require a double-blinded, placebo-controlled open food challenge.
Agreement percentage: 90.97%
Clinical diagnostic suspicion of CMPA must be confirmed through total allergen elimination, i.e., the exclusion of the CMPs, the complete disappearance of the attributed symptoms, and the later performance of the oral food challenge (OFC), which can be open, blinded, or double-blinded, depending on the symptoms, medical history, and age of the child.38,74 Currently, the best strategy for diagnosing CMPA, as well as any food allergy, is the performance of a double-blinded placebo-controlled food challenge (DBPCFC), given that when the DBPCFC is performed, the results are negative, in 70% of patients with positive OFC results.75 However, the DBPCFC is difficult to perform, is time-consuming and costly, and so is reserved for research studies. Thus, the open OFC is accepted as the diagnostic confirmation standard in the context of daily clinical practice, especially in young children, because there are objective symptoms during the test. With respect to subjective symptoms, such as food rejection, nausea, headache, etc., the performance of a DBPCFC is required.73 The OFC, and especially the DBPCFC, are useful for suspending unnecessary elimination diets that can have nutritional repercussions.73,76
Statement 8. The open food challenge should be carried out after a CMP elimination diet and on an asymptomatic patient. The elimination period before performing the challenge test is 1 to 2 weeks in IgE-mediated allergies and 2 to 4 weeks in non-IgE-mediated allergies. With respect to more severe forms, the waiting period should last until there is total clinical normalization.
Agreement percentage: 95.83%
The open OFC is the most widely utilized test in daily practice. In the challenge, once the patient has been on a CMP elimination diet and is asymptomatic, he/she is exposed to the allergen again. Both the physician and the parents are aware that the child is receiving the CMP, to confirm the diagnosis of CMPA. There are no studies providing evidence on the length of time the allergen should be eliminated from the diet. According to expert consensus, the elimination period is 1 to 2 weeks for IgE-mediated allergies and 2 to 4 weeks for non-IgE-mediated allergies.31,37 Preferably, in patients with IgE-mediated symptoms, the test should be performed in the hospital, but when delayed non-IgE-mediated allergic reactions are expected, with gastrointestinal symptoms, such as chronic diarrhea, colitis, allergic proctocolitis, GERD, etc., it can be performed in the outpatient setting, at the patient’s home, always with the provision of emergency indications.73
Statement 9. The challenge test in IgE-mediated CMPA should be indicated and supervised by trained medical personnel in the hospital ward or outpatient care center.
Agreement percentage: 97.91%
There are protocols for performing the OFC. The DRACMA guideline panel gives the following recommendations for carrying out the challenge in the context of IgE-mediated CMPA, stating that it should always be indicated and supervised by trained medical personnel when performed in the hospital:19,73,74
- 1
Calculate the total dose according to the maximum quantity consumed per serving or based on the total weight of the patient.
- 2
Use the same type of milk that the patient will consume every day, in case of a negative challenge.
- 3
Start with one drop or a 0.1 ml dose. Give a dose every 20-30 minutes, increasing it logarithmically, e.g., 0.1, 0.3, 1.0, 3.0, 10, 30, and 100 ml. In cases at high risk for anaphylaxis, more diluted doses could be started, and the time between doses should be longer, even up to 1 h.77
- 4
Discontinue the procedure at the first onset of objective symptoms, which include generalized urticaria, erythematous rash with pruritus, vomiting, abdominal pain, nasal congestion, repetitive sneezing, watery rhinorrhea, rhino-conjunctivitis, stridor, changes in tone of voice, laryngospasm, inspiratory stridor, cough, pallor, changes in behavior, tachycardia, hypotension, collapse, and anaphylaxis.
- 5
Clinical observations will be carried out for 2 to 4 h after the last dose of milk is received.
- 6
If there is no symptomatology, the patient should receive at least 200 ml of CMP daily for at least 2 weeks, after which CMP should no longer be restricted.
Statement 10. The challenge test in mild or moderate non-IgE-mediated CMPA can be carried out at home. In children with FPIES, the test should be performed under the supervision of trained medical personnel.
Agreement percentage: 97.91%
The OFC can be carried out at home in cases of mild-to-moderate clinical symptoms, as long as the family is in agreement, given that they are the ones that must be trained for its correct performance and control. The challenge test should not be carried out at home in patients with severe clinical symptoms, FPIES, clinical suspicion of an IgE-mediated mechanism, and/or in patients with positive CMP-specific IgE tests. For breastfed children, cow’s milk should be reintroduced into the maternal diet, starting with one daily serving of milk or dairy products the first week, and if the infant does not present with symptoms, progressively increase the quantity of milk or dairy products on a weekly basis. The possible appearance of symptoms should be looked for up to 4 weeks after the reintroduction. If symptoms reappear, all CMP use in the maternal diet must be suspended.37 In formula-fed children, a measure of the special formula should be substituted by a measure of CMP formula every day, in at least 2 of the daily bottle feedings. If the symptoms do not reappear once the change in those two feedings is complete, a full bottle of special formula can be substituted by a full bottle of CMP formula, until the total reintroduction is complete.37 Preferably, a new food should not be introduced into the diet while the challenge is being carried out, to avoid confusions. During the challenge, if no symptoms appear, the observation period following the reintroduction of CMP into the diet should be 2 to 4 weeks.37
The 2018 Brazilian consensus recommends that all OFCs be carried out under medical supervision, even in children whose gastrointestinal clinical manifestations are mild or moderate, delayed, or non-IgE-mediated. In those types of cases, the challenges can be performed at outpatient care centers.22
Statement 11. A previous anaphylactic reaction to CMP ingestion is a contraindication for the oral food challenge. The test should not be carried out in patients with life-threatening clinical manifestations.
Agreement percentage: 100%
In the case of patients with manifestations of anaphylaxis, the OFC is not required, given that it can involve risk, and specific IgE determination can be used as a diagnostic alternative.38
Statement 12. Specific IgE tests for CMP indicate sensitization, but not necessarily CMPA. Negative specific IgE tests for CMP do not exclude the diagnosis of CMPA in patients in whom there is clinical suspicion.
Agreement percentage: 100%
Statement 13. Skin prick tests and specific IgE blood tests only have diagnostic value in patients with clinical symptoms consistent with IgE-mediated CMPA or in children with a positive oral food challenge.
Agreement percentage: 97.91%
Statement 14. Skin prick tests or specific IgE in a blood sample are not recommended for diagnostic confirmation of CMPA in children.
Agreement percentage: 100%
A positive SPT to cow’s milk indicates sensitization to CMPs and the presence of an IgE-mediated immunologic process, but it should always be interpreted in the clinical context.38 The diameter of a wheal > 8 mm is very suggestive of a CMPA diagnosis, but it should always be analyzed within the clinical context of the patient.78 In clinical practice, when an OFC is always performed to confirm the diagnosis of CMPA, a SPT is not required. Nevertheless, SPTs can aid in avoiding an OFC in certain patients. In patients with a high probability of IgE-mediated CMPA that have a positive SPT ≥ 3 mm, the OFC would not be recommended and the diagnosis of CMPA would be made, but close to 5-6% would be false positives.73 The level of evidence is low and requires more studies, before being able to widely recommend carrying out the SPT. SPTs for other allergens should only be ordered if the child presents with immediate-onset symptoms, when the suspect allergen is incorporated during complementary feeding.59
A positive specific IgE test indicates sensitization, but not necessarily allergy, and so correlation with the patient’s clinical history is required.79 Children with gastrointestinal manifestations of CMPA have a greater probability of having a negative specific IgE test, compared with those that have dermatologic manifestations. A negative IgE test alone does not rule out the diagnosis of CMPA.38 In clinical practice, when an OFC is always performed for diagnosing CMPA, CMP-specific IgE quantification is not required. Nevertheless, IgE testing can aid in avoiding an OFC in certain cases. In patients with a very high probability of presenting with IgE-mediated CMPA that have a positive CMP-specific IgE test > 0.7 IU/l, the OFC would not be recommended and CMPA would be diagnosed, albeit 2-5% would be false positives.73 High levels of specific IgE predict failure for achieving desensitization.80,81 Importantly, there are discrepancies in specific IgE values due to different assays, signifying that the predictive values determined by one method should not be applied to those carried out by other methods.82–85 Therefore, even though CMP-specific IgE determination has some usefulness, it is not indispensable, and is not systematically recommended for diagnosing CMPA, in addition to the fact that it can be costly. Total IgE determination or the specific IgE/total IgE ratio are not superior to specific IgE in the diagnostic approach to CMPA, and so should not be utilized.86
Statement 15. Neither the determination of specific IgG nor the patch test, nor a complementary laboratory test, nor gastrointestinal endoscopy is recommended for the diagnostic confirmation of IgE-mediated or non-IgE-mediated CMPA.
Agreement percentage: 99.3%
The determination of other complementary laboratory tests or the performance of gastrointestinal endoscopy are not needed to confirm the diagnosis of IgE-mediated or non-IgE-mediated CMPA. The decision to include some of those tests for making the differential diagnosis should be made by a pediatric gastroenterologist.
There has been a lack of clarity and evidence on the usefulness of IgG determination in the diagnosis of food allergies, including CMPA, for over three decades.87–90 IgG4 has structural characteristics that promote anti-inflammatory activity and it is frequently considered a mediator of tolerance to allergens.91 Recent studies have related it to EoE, but the pathogenic relation has not yet been established.92,93 The significance of the IgGs or the IgG subclasses in CMPA is not well understood and is controversial. Thus, at present, its determination plays no role in the diagnosis of CMPA.38,73,94
Patch tests can be useful, albeit limited, for diagnosing CMPA with non-IgE-mediated manifestations, or those that are considered delayed reactions.95,96 Their use is not standardized and there are only a few well-designed studies for demonstrating their clinical applicability.97,98 They have been used in the diagnosis of food allergies, including CMPA, in patients with AD.99 Patch tests alone are not sufficient for diagnosing CMPA.73,100 Together with the SPTs, they can aid in ruling out CMPA in children that have allergic manifestations, given that they have 100% predictive values when their use is combined.101 However, if a patch test is positive, it must always be correlated with the clinical manifestations and confirmed by an OFC.102 Given the lack of standardization in its performance, as well as the difficulty in reading the test and its subjectivity, its use alone, or systematically, is not recommended for diagnosing CMPA.94
Currently, there is insufficient evidence to support the value of fecal calprotectin determination in making the diagnosis of CMPA and its differential diagnosis, and therefore, it is not recommended as part of the approach.103 There is still little evidence on the usefulness of ultrasound as the complementary imaging study for diagnosing CMPA, in the context of the approach to an infant with hematochezia,104–106 and so its use cannot yet be recommended for making the CMPA diagnosis. Many of the patients that undergo an endoscopic procedure in the diagnostic approach to GERD that is not responding to treatment, as well as a colonoscopic study in the approach to rectorrhagia and hematochezia, may present with endoscopic and histopathologic findings consistent with CMPA.107 Upper and lower endoscopy should be considered in patients with persistent gastrointestinal symptoms, growth failure, or iron-deficiency anemia, but with the understanding that macroscopic and histologic findings, such as mucosal atrophy or eosinophilic infiltrates are not sensitive or specific for CMPA.38,73,108 Therefore, the systematic performance of those studies for diagnosing CMPA is not required, and should only be carried out in patients whose symptomatology is not resolved through an elimination diet, or when an alternative diagnosis is strongly suspected.59
Module 3. Treatment of CMPAThe diagnostic elimination diet is based on the complete exclusion of the allergenic proteins (CMPs) from the diet, to reverse the clinical manifestations of CMPA. After the clinical and nutritional recovery of the patient, an OFC should be performed to confirm the diagnosis of CMPA. Once confirmed, the therapeutic elimination diet should be started and continued until oral tolerance has been developed.17,22,38,66,73,94,109–113
Statement 16. An elimination diet that excludes allergenic proteins continues to be the conduct of choice for controlling the clinical manifestations of CMPA.
Agreement percentage: 99.3%
A CMP-free diet should be adopted to control the clinical manifestations of CMPA, and the same principle holds true for other food allergies. The elimination of CMPs from the diet leads to the cure, which occurs when the patient develops oral tolerance to CMPs. The time it takes to achieve tolerance varies greatly and depends on the mechanisms involved in the development of the allergy. In infants, with non-IgE-mediated reactions to CMPs and gastrointestinal tract involvement, it occurs in the majority of cases after 6 months of elimination treatment, at one year of age, and not over 3 years of age.17,22,38,66,73,94,109–111 In the patients with IgE-mediated CMPA, acquiring tolerance is generally delayed. Testing to determine whether the patient has developed oral tolerance is recommended every 6 or 12 months.17,22,38,66,73,94,109–112
Statement 17. At present, there is no therapeutic alternative for accelerating the development of oral tolerance
Agreement percentage: 97.22%
Despite innumerable theories and efforts in the fields of basic and clinical research, there is still no alternative with practical efficacy that can be used to accelerate the development of oral tolerance.17,38,66,73,94 A greatly relevant aspect is the quality of the elimination diet provided to the infants with CMPA and their mothers.59,109–111 When the correct allergen elimination diet does not result in clinical recovery, accepted practice is to revise the diet, and if necessary, rule out the CMPA diagnosis.17,22,38,66,73,94,109–112
Statement 18. The treatment of CMPA in exclusively breastfed children is the elimination of CMP in the maternal diet. Mothers should receive supplementation with 1 g/day of calcium and 600 IU/day of vitamin D.
Agreement percentage: 99.3%
In exclusively breastfed infants that present with clinical manifestations consistent with CMPA, the elimination of CMPs (milk derived from and prepared with cow’s milk or milk from other mammals) from the maternal diet should be recommended.17,38,59,112 The elimination diet is the same one that enabled the diagnosis of CMPA.31 Women on a CMP elimination diet that are breastfeeding should receive a supplement of 1.0 g of calcium per day and 600 IU/day of vitamin D.37,38,59,109 There are no clinical indicators that suggest the need to exclude other proteins from the diet of the breastfeeding mother. However, reactions, especially to soy and egg proteins, could be transmitted through the breast milk,37 as well as dry fruit and wheat proteins, albeit with much less frequency.59 If it were necessary to exclude numerous foods, the mother should be evaluated by a nutritionist who guides her diet, to prevent any nutrient deficiency.37,59,109
Statement 19. The treatment for CMPA in children that are both breastfed and CMP formula-fed is the elimination of CMP from the maternal diet and substitution of the formula with extensively hydrolyzed CMP.
Agreement percentage: 99.3%
The diet of choice essentially depends on the onset of clinical manifestations, i.e., if they began during breastfeeding or after complementary feeding or the incorporation of a CMP formula.37,38,59,66 If the clinical manifestations began during breastfeeding, CMPs should be eliminated from the maternal diet, with the maximum effort made to continue breastfeeding. If it is necessary to supplement breastfeeding, regular cow’s milk-based formula should be suspended and an extensively hydrolyzed protein formula (eHPF) prescribed.
Statement 20. If CMPA is produced only after the introduction of foods or conventional CMP formula, the mother does not need to suspend CMP from her diet, during the entire treatment.
Agreement percentage: 95.13%
If there were no clinical manifestations of CMPA during exclusive breastfeeding, and they began after the introduction of foods or infant CMP formula, the recommendations are to eliminate CMPs from the maternal diet, with the maximum effort made to continue breastfeeding, until the clinical manifestations disappear. After controlling the clinical manifestations, while breastfeeding is still in effect, the mother should resume her usual CMP consumption. CMP elimination in her diet is not necessary if the symptoms do not reappear. The supplemental formula utilized in feeding the infant should be an eHPF and the CMP formula should be suspended. If the mother must follow a CMP elimination diet, she should receive dietary advice, especially with regard to supplements of calcium and other nutrients.37,38,59,66,109
Statement 21. Extensively hydrolyzed formulas are safe, hypoallergenic, and nutritionally adequate. They are the first choice in the treatment of infants with CMPA that are not exclusively breastfed.
Agreement percentage: 99.3%
For infants with CMPA that are fed with CMP-based infant formula, its suspension is recommended, and if necessary, its substitution by an adequate formula. A formula is considered adequate because it has been tested and is known to be tolerated by over 90% of the patients with CMPA, with a 95% confidence interval. The tests are conducted on patients with IgE-mediated CMPA and the results are extrapolated to all patients, including those with cell-mediated CMPA. The eHPF contains more than 85% of peptides with a molecular weight lower than 1500 Da. It is suitable for the treatment of CMPA and is recommended as the first option.17,22,37,38,66,73,94,109–113 Those criteria are met by the extensively hydrolyzed serum and/or casein formulas, as well as by hydrolyzed rice formulas (HRFs) and amino acid-based formulas (AAFs).17,22,38,66,73,94,109–111 In infants whose nutritional status has been affected, with clinical symptoms consistent with enteropathy, an eHPF with medium-chain triglycerides and no lactose is recommend, up until recovery from the intestinal lesion.37,56 However, there is no support for the systematic removal of lactose and it should only be carried out in patients that have transitory intolerance due to enteropathy. In recent years, there has been interest in the possible role of probiotics in the development of oral tolerance. Nevertheless, double-blinded clinical trials are needed to confirm that hypothesis.17,37,109,114
The broad spectrum of clinical presentation and severity in cases of CMPA, the different infant formulas available, the difficulties in confirming the diagnosis, and the need for an elimination diet and follow-up lasting several months are all obstacles for conducting randomized trials. Prospective clinical trials, some of which are randomized, that evaluate safety, hypoallergenicity, palatability, and infant growth (weight and length), with the use of new formulas for infants with CMPA, can be found in the literature. Their results show differences in palatability (better results for extensively hydrolyzed serum protein formulas), but no significant differences in the other aspects analyzed.109,115 The pharmacoeconomic model developed in Brazil compared two possibilities: starting treatment (diagnostic elimination diet) with an eHPF or AAF, both followed by an OFC. After diagnostic confirmation, the elimination diet was continued, with an eHPF. The model showed cost savings and reduced duration of the symptomatic period.116 Currently, the majority of guidelines recommend that eHPFs initially be used in the diagnostic elimination diet. If the clinical manifestations do not remit within 2 weeks, the recommendation is to exclude the eHPFs and substitute them with AAFs. The patients should recover in a maximum of 2 to 4 weeks. AAFs are considered 100% effective for controlling the clinical manifestations of CMPA. Therefore, the persistence of clinical manifestations during an exclusion diet with an AAF should prompt new studies to explain the clinical manifestations and rule out the diagnosis of CMPA.17,22,37,38,66,73,94,109–112
Statement 22. The amino acid formula is recommended as a first option in the treatment of patients with severe forms of CMPA
Agreement percentage: 99.3%
For patients with severe conditions of CMPA, several guidelines recommend starting the elimination diet with an AAF, which has greater therapeutic efficacy. It is especially recommended in patients with anaphylaxis, FPIES, and eosinophilic diseases, and even though there is not total agreement, it can also be considered in severe enteropathy with diarrhea, malnutrition and/or delayed growth, and hypoproteinemia.17,22,37,38,66,73,94,109–112 Regarding anaphylaxis, long-term management should include a challenge with an eHPF before reintroducing CMP, and should be carried out after 6-9 months, or when the infant is one year old, always in a hospital setting, under medical supervision.113
Statement 23. Soy infant formula can be considered a management option in children above 6 months of age with IgE-mediated CMPA that are not exclusively breastfed.
Agreement percentage: 94.44%
Soy infant formula can be considered a management option in children that are not breastfed. According to the ESPGHAN guidelines, it can be an alternative in the elimination diet from 6 months of age, in infants with IgE-mediated CMPA, with no gastrointestinal involvement.17,22,38,66,73,94,109–111 HRFs are less costly than eHPFs, more palatable, and more accepted by vegan/vegetarian families. Current formulas have shown a good level of safety, the arsenic content is within the allowed limits, and they do not contain phytosteroles.109,117 If an eHPF is not available, if the infant refuses to drink it, or if it is not affordable, the HRF can be considered a second option, or the soy formula can be an option, in infants above 6 months of age that present with IgE-mediated CMPA.73–113
Statement 24. Partially hydrolyzed formulas, milk from other mammals, and plant-based beverages should not be used as part of the treatment for CMPA.
Agreement percentage: 100%
Neither a formula that is lactose-free, with intact protein, nor a formula that is partially hydrolyzed is adequate for treatment because they can trigger symptoms in sensitized infants due to the presence of protein or larger peptides. Substitution with the milk of other mammals is not recommended because of the high incidence of cross-reactions in patients with CMPA.17,22,37,38,66,73,94,109–113 Plant-based beverages (inadequately called “milks”, given that they do not come from mammary glands) made from almonds, hazelnuts, rice, and soy are not indicated for infants, given that they do not provide the necessary nutrients.109,118
Statement 25. Complementary feeding should be started at the same age as in children without CMPA. The inclusion of foods should follow the same recommendations made for children without allergies.
Agreement percentage: 100%
Once the diagnosis of CMPA is made, the elimination diet should be followed for at least 6 months. During the elimination diet, the infant should start or continue receiving complementary foods. Complementary feeding should begin at the same age it is started in children that do not have CMPA and the inclusion of foods should follow the same recommendations that apply to children without CMPA. The introduction of foods considered allergenic, such as eggs, fish, wheat, and other proteins, should not be delayed in children with CMPA.37,59 The quantities of macronutrients and micronutrients that children receive in the special formula and complementary foods should be carefully assessed, as well as the prescription of supplements, when necessary, with special attention in relation to calcium, iron, zinc, vitamin D, and vitamin A intake.17,22,37,38,66,73,94,109–113
Statement 26. The challenge test to confirm tolerance recovery can be performed at home for the non-IgE-mediated forms of CMPA, after at least 6 months of treatment and/or having reached one year of age.
Agreement percentage: 99.3%
After the diagnosis is confirmed, the patient must have no exposure to the allergens, so that he/she can remain asymptomatic, grow and develop normally, and acquire oral tolerance. There is no proven method for accelerating the development of oral tolerance. The evaluation of the persistence or resolution of CMPA can only be established through testing for acquired tolerance, which involves the controlled reintroduction of the CMP, under medical supervision. The OFC can be performed for two purposes: 1) to confirm the diagnosis, and 2) to characterize the development of oral tolerance. It can be performed after 6 to 12 months of treatment. Another possibility, in the case of infants, is to perform the test for confirming the development of oral tolerance when the patient reaches one year of age.17,22,38,66,73,94,109–111 In mild forms of gastroesophageal reflux, colic, constipation, and proctocolitis, tolerance can develop early, within 3-6 months, whereas in FPIES and IgE-mediated reactions, it develops later. Thus, testing should be held off until 12, 18, or even 24 months, in the most severe cases.37
In children with non-IgE-mediated CMPA, the OFC can be performed at home, with baked dairy products or small portions of cow’s milk, as indicated in the diagnostic OFC.37
Statement 27. In breastfed children, starting the test by incorporating dairy products into the maternal diet, to then be incorporated into the diet of the infant, is recommended.
Agreement percentage: 97.91%
In breastfed children, the guidelines recommend starting the tolerance test by incorporating dairy products into the maternal diet, to then be incorporated into the diet of the infant, despite the fact that there are no data on its benefits.59,66
Statement 28. To confirm tolerance in children with IgE-mediated CMPA, FPIES, or severe forms of CMPA, the performance of specific IgE tests should be performed before the challenge test, after 12 or more months of treatment, and under the supervision of trained medical personnel.
Agreement percentage: 97.22%
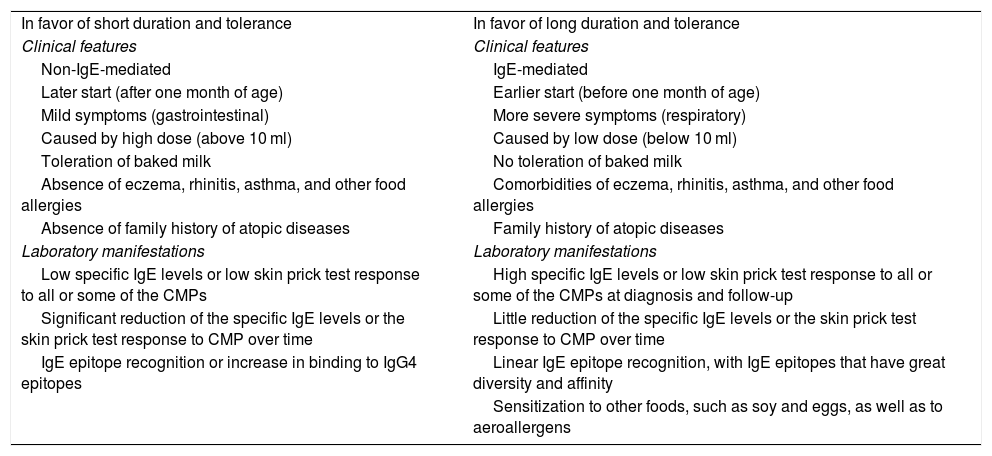
In patients with IgE-mediated CMPA or FPIES that can be associated with IgE allergies, the need for carrying out a CMP-specific IgE blood test or CMP-specific IgE skin test, once the period of treatment is over, and before the CMP challenge is performed, should be considered. In those patients, the OFC be carried out when those tests are negative and under the supervision of trained medical personnel, utilizing the same procedure as in the diagnosis.37,56,66 In cases of unfavorable response to the reintroduction of CMPs, acquired tolerance should be periodically re-evaluated every 6-12 months, under medical supervision, with respect to the characteristics of each case and the severity of the response in the previous test.37,56 Different risk factors for the delay in acquired tolerance have been described (Table 3).119
Factors related to the tolerance and persistence of cow’s milk protein allergy.
| In favor of short duration and tolerance | In favor of long duration and tolerance |
| Clinical features | Clinical features |
| Non-IgE-mediated | IgE-mediated |
| Later start (after one month of age) | Earlier start (before one month of age) |
| Mild symptoms (gastrointestinal) | More severe symptoms (respiratory) |
| Caused by high dose (above 10 ml) | Caused by low dose (below 10 ml) |
| Toleration of baked milk | No toleration of baked milk |
| Absence of eczema, rhinitis, asthma, and other food allergies | Comorbidities of eczema, rhinitis, asthma, and other food allergies |
| Absence of family history of atopic diseases | Family history of atopic diseases |
| Laboratory manifestations | Laboratory manifestations |
| Low specific IgE levels or low skin prick test response to all or some of the CMPs | High specific IgE levels or low skin prick test response to all or some of the CMPs at diagnosis and follow-up |
| Significant reduction of the specific IgE levels or the skin prick test response to CMP over time | Little reduction of the specific IgE levels or the skin prick test response to CMP over time |
| IgE epitope recognition or increase in binding to IgG4 epitopes | Linear IgE epitope recognition, with IgE epitopes that have great diversity and affinity |
| Sensitization to other foods, such as soy and eggs, as well as to aeroallergens |
CMPs: cow’s milk proteins; IgE: immunoglobulin E; IgG4: immunoglobulin G subclass 4.
The symptoms of IgE-mediated CMPA are diverse and affect a variety of organs and systems. They more frequently involve the skin and mucosae, and less frequently affect the gastrointestinal and respiratory tracts. CMPA cause by mixed mechanisms (IgE-mediated and non-IgE-mediated) manifests as cutaneous symptoms, specifically AD, and the non-IgE-mediated reaction has late-onset symptoms that are predominantly gastrointestinal, including three syndromes: proctocolitis, enteropathy, and FPIES, or with symptoms that resemble functional gastrointestinal disorders: infant colic, infant regurgitation, and constipation. The most frequent clinical presentation of CMPA in exclusively breastfed children is proctocolitis.
The open OFC is considered the first choice for confirming the diagnosis of CMPA. From a clinical perspective, the DBPCFC may be necessary in selected patients.
The elimination diet for excluding allergenic proteins continues to be the conduct of choice for controlling the clinical manifestations of CMPA. At present, there is no other therapeutic alternative that enables the development of oral tolerance. The treatment for CMPA in exclusively breastfed children is the elimination of CMPs in the maternal diet. In non-breastfed children, eHPFs are the first choice in the treatment of infants with CMPA. AAFs are recommended as the first option in the treatment of patients with severe forms of the condition, mainly anaphylaxis and FPIES, or in cases in which eHPFs fail to reverse the symptoms of CMPA. The evaluation of the persistence or resolution of CMPA can only be established through testing for acquired tolerance, which involves the reintroduction of the CMP, under medical supervision.
Ethical considerationsThis document is a consensus, with no patient participation, and therefore no statements of informed consent for receiving treatment or participating in research were required. The present work is not a study with participating patients, and so involves no breach of the current bioethical research regulations and requires no authorization by an institutional ethics committee. Because this work is a consensus that is not conducted on patients, nor does it utilize any personal patient information, no patient can be identified. The article contains no images or data relating to any patients.
Financial disclosureNo financial support of any type was needed regarding the development of the present consensus.
Conflict of interestMCT: I have received fees and funds to attend congresses from Danone Nutricia, Sanofi, Nestlé, and Mead Johnson.
MBM: I have received fees for being a consultant and for conferences and have received funds to attend scientific events from Danone Nutricia, Nestlé, RB Mead Johnson, Ache-Biogaia, and Bago.
RVF: I have received fees for conferences and have received funds to attend congresses, from Abbott Farmacéutica, BioGaia, Carnot, Columbia, Danone, Kellogg’s, Medix, Nestlé, Nestlé Nutrition Institute, Pfizer Farmacéutica, Sanofi, and Takeda México. I have received fees for being a consultant at Carnot, Nestlé, and Sanofi.
DJBC: No conflict of interest.
CGBM: I have received funds to attend congresses and received fees for dissertations as KOL from Nutricia, Mead Johnson, Nestlé, Sanofi, Ethical Nutrition, and Biocodex.
LDC: No conflict of interest.
MMHC: No conflict of interest.
LL: I have received fees for conferences and have received funds to attend congresses from the Nestlé Nutrition Institute, Abbott Nutrition, Pfizer Nutrition, Nutricia, Mead Johnson Nutrition, Nestlé Nutrition, Actelion. I have received for being a consultant at Reckitt Benchiser.
SM: No conflict of interest.
GCM: I have received funds from Nutricia, Bago, and Mead Johnson to attend congresses.
GJO:I have received funds from Nutricia Bago and Mead Johnson to attend congresses.
LROP: No conflict of interest.
COP: No conflict of interest.
JPRL: I have received fees for conferences from Danone Nutricia, Farma de Colombia, Abbott EPD, Abbott Nutrition.
PCS: I have received fees from Nutricia for conferences and being on the advisory board.
NCVP: No conflict of interest.
Please cite this article as: Toca MC, Morais MB, Vázquez-Frias R, Becker-Cuevas DJ, Boggio-Marzet CG, Delgado-Carbajal L, et al. Consenso sobre el diagnóstico y el tratamiento de la alergia a las proteínas de la leche de vaca de la Sociedad Latinoamericana de Gastroenterología, Hepatología y Nutrición. Rev Gastroenterol Méx. 2022;87:235–250.