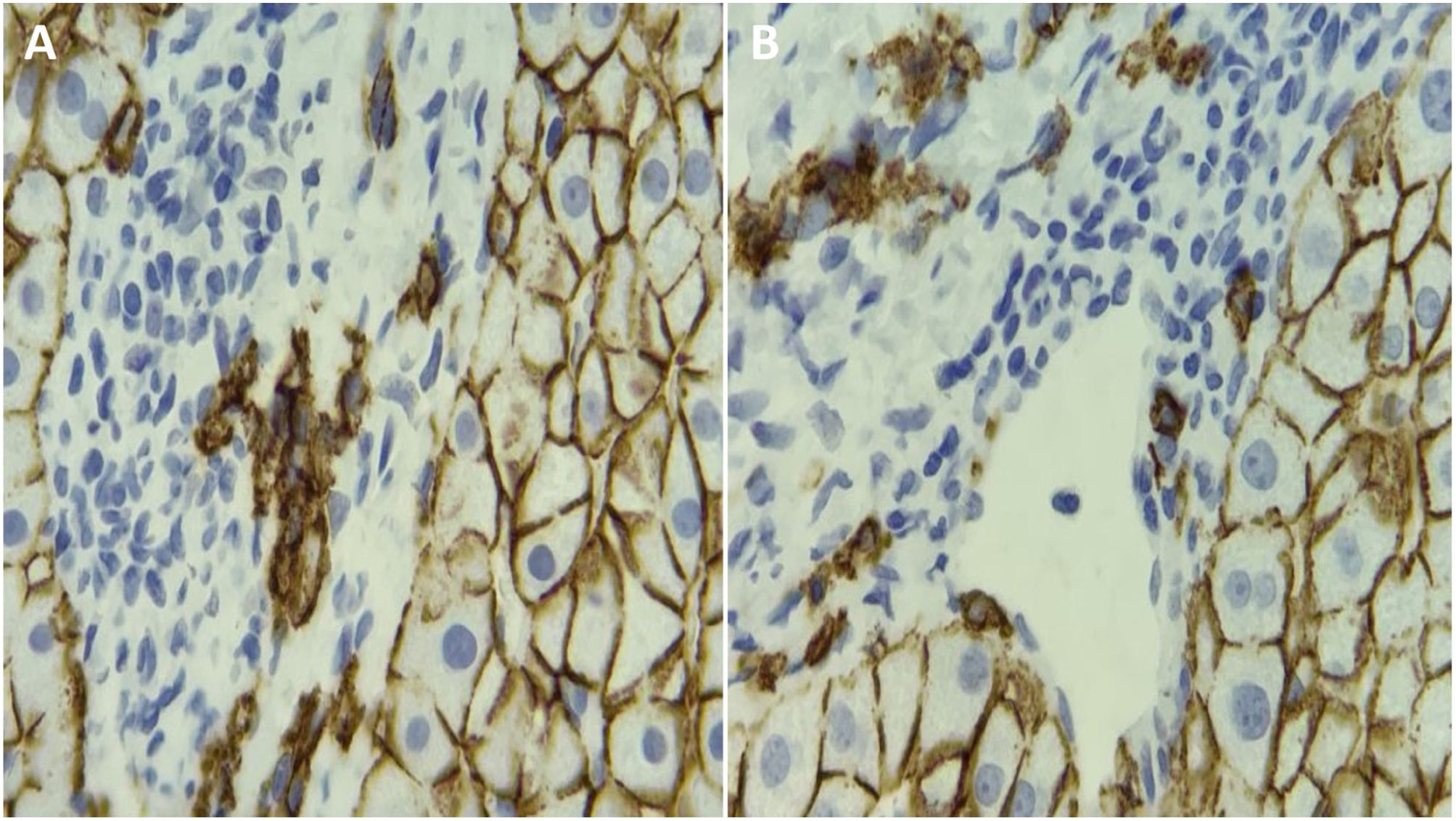
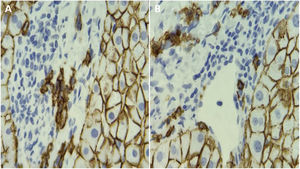
Autoimmune hepatitis (AIH) is associated with periportal infiltration by plasma cells. Plasma cell detection is routinely performed through hematoxylin and eosin (H&E) staining. The present study aimed to assess the utility of CD138, an immunohistochemical plasma cell marker, in the evaluation of AIH.
Materials and methodsA retrospective study was conducted, in which cases consistent with AIH, within the time frame of 2001 and 2011, were collected. Routine H&E-stained sections were used for evaluation. CD138 immunohistochemistry (IHC) was performed to detect plasma cells.
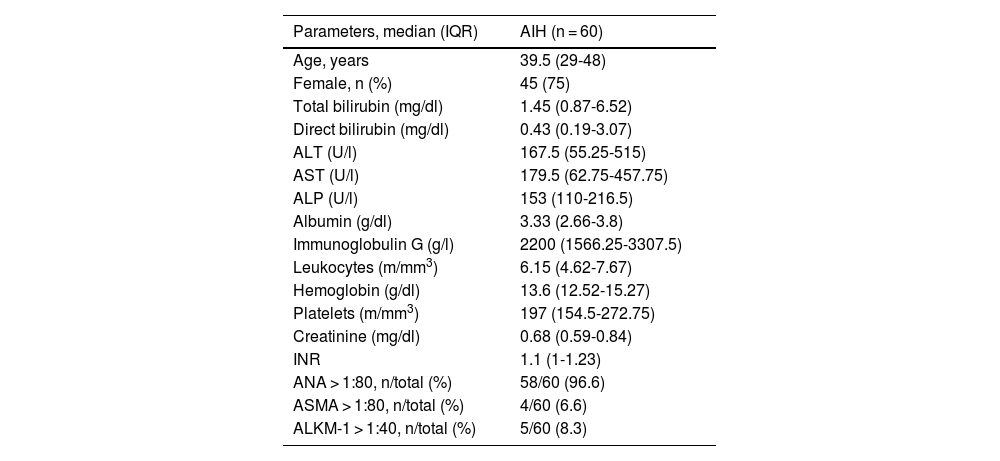
ResultsSixty biopsies were included. In the H&E group, the median and interquartile range (IQR) was 6 (4-9) plasma cells/high power field (HPF) and was 10 (IQR 6-20) plasma cells/HPF in the CD138 group (p < 0.001). There was a significant correlation between the number of plasma cells determined by H&E and CD138 (p = 0.31, p = 0.01). No significant correlation was found between the number of plasma cells determined by CD138 and IgG level (p = 0.21, p = 0.09) or stage of fibrosis (p = 0.12, p = 0.35), or between IgG level and stage of fibrosis (p = 0.17, p = 0.17). No significant correlation was found between the treatment response and the number of plasma cells determined by H&E (p = 0.11, p = 0.38), CD138 (p = 0.07, p = 0.55), or stage of fibrosis (p = 0.16, p = 0.20). CD138 expression was different between the treatment response groups (p = 0.04).
ConclusionCD138 increased the detection of plasma cells in liver biopsies of patients with AIH, when compared with routine H&E staining. However, there was no correlation between the number of plasma cells determined by CD138 and serum IgG levels, stage of fibrosis, or response to treatment.
La hepatitis autoinmune (HAI) está asociada con infiltración periportal de células plasmáticas. La detección de células plasmáticas se realiza rutinariamente por medio de tinción hematoxilina-eosina (HE). El objetivo del presente estudio fue evaluar la utilidad de CD138, un marcador inmunohistoquímico de células plasmáticas, en la evaluación de HAI.
Materiales y métodosEstudio retrospectivo, los casos compatibles con HAI se recolectaron de 2001 a 2011. Se utilizó la tinción HE para evaluación y CD138 para detectar células plasmáticas.
ResultadosSe incluyeron 60 biopsias. En el grupo HE, la mediana y el rango intercuartil (RIQ) fue 6 (4-9) células plasmáticas/campo de alto poder (CAP) y de 10 (RIQ 6-20) células plasmáticas/CAP en el grupo CD138 (p < 0.001). Existió una correlación significativa entre el número de células plasmáticas determinado por HE y CD138 (p = 0.31, p = 0.01). No se encontró relación significativa entre el número de células plasmáticas determinadas por CD138 y el nivel IgG (p = 0.21, p = 0.09) o el estadio de fibrosis (p = 0.12, p = 0.35), o entre el nivel de IgG y el estadio de fibrosis (p = 0.17, p = 0.17). No se encontró correlación significativa entre la respuesta al tratamiento y el número de células plasmáticas determinadas por HE (p = 0.11, p = 0.38), CD138 (p = 0.07, p = 0.55), o el estadio de fibrosis (p = 0.16, p = 0.20). La expresión de CD138 fue diferente entre los grupos de respuesta al tratamiento (p = 0.04).
ConclusiónEl CD138 incrementó la detección de células plasmáticas en biopsias hepáticas de pacientes con HAI, en comparación con la tinción de HE de rutina. Sin embargo, no existió correlación entre el número de células plasmáticas determinadas por CD138 y los niveles de IgG en suero, el estadio de fibrosis o la respuesta al tratamiento.
Autoimmune hepatitis (AIH) is a disease of unknown etiology associated with persistent inflammation of the liver. It typically affects females and is characterized by elevated transaminases, the presence of autoantibodies, and increased serum levels of immunoglobulin G (IgG).1,2 Biochemical and immunologic findings in those patients are insufficient for making the diagnosis, and a liver biopsy is needed.1 The most typical finding in the liver biopsy is the presence of interface hepatitis. In addition, there is usually a lymphoplasmacytic portal infiltrate.3,4 Reports state that up to 34% of patients have no plasma cells, and said percentage is unknown in Mexican patients.5 Plasma cell determination through hematoxylin and eosin (H&E) staining is routinely carried out, whereas the use of CD138 (an immunohistochemical plasma cell marker) in the evaluation of AIH has not been widely assessed. Most hepatologists consider the presence of plasma cells to be virtually diagnostic of AIH, but their prognostic implications are largely unknown.1
The present study aimed to assess the performance of CD138 immunohistochemistry (IHC) in the histopathologic diagnosis of AIH, by comparing the number of plasma cells found through H&E staining versus CD138 staining. A secondary objective was to study the association between the number of plasma cells determined by CD138 IHC and serum IgG levels, stage of fibrosis, and response to treatment.6
Materials and methodsA cross-sectional retrospective study was conducted. Eligible patients were those with a histopathologic diagnosis of AIH made at the National Institute of Medical Sciences and Nutrition Salvador Zubirán, within the time frame of 2001 to 2011. We included patients in which the clinical, serologic, and histologic findings were all consistent with definitive or probable AIH, according to the revised score of the International Autoimmune Hepatitis Group (IAIHG).7,8 Patients were tested for IgG, antinuclear antibodies (ANA), anti-smooth muscle antibodies (ASMA), and anti-liver-kidney microsomal type 1 antibodies (ALKM-1). A serum titer of 1:80 or higher was considered positive for ANA and ASMA, and a titer of 1:40 or higher was considered positive for ALKM-1. We excluded patients whose paraffin blocks were missing, patients whose medical records were incomplete, patients that had received previous treatment, patients with a concomitant liver disease, or patients whose diagnosis was made in liver transplant specimens.
We cut 4 um sections from the formalin-fixed tissue embedded in paraffin blocks and performed routine H&E staining and immunostaining for CD138 on all patients. All sections were reviewed by an experienced gastropathologist (P.R.M. and J.S.R.), in a blinded fashion. The number of portal spaces was determined at low power (x4); plasma cells were counted at high power (x40) in both the H&E and CD138-stained slides. In addition, inflammation grade and fibrosis stage were determined according to the Batts and Ludwig system.9
All patients were treated similarly at the discretion of the hepatologist. Treatment response was defined as complete, incomplete, or as treatment failure, according to IAIHG criteria. Complete response was regarded as biochemical remission (normalization of serum AST, ALT, and IgG levels); incomplete response as insufficient improvement of laboratory test findings to satisfy the criteria for complete response; and treatment failure as worsening laboratory test results, despite compliance with standard therapy.7,10
Statistical analysisContinuous variables were expressed as median and interquartile range (IQR). The Wilcoxon signed-rank test was used to compare the number of plasma cells determined by H&E staining versus CD138 staining. The Kruskal-Wallis test was used to compare the number of plasma cells determined by CD138 IHC and treatment response. A 2-tailed p value of <0.05 was considered statistically significant. The Spearman’s correlation coefficient (p) was used to evaluate the relationship between continuous or discrete variables and a correlation coefficient greater than 0.30 was considered positive. Correlations were computed based on the number of pairs with non-missing data. In the descriptive variables, the number of non-missing values was used. In the calculation of frequencies, missing values were excluded, and percentages were based on the number of non-missing values.
Ethical considerationsThe present research fulfilled the current regulations on bioethical research. Patient consent for publication was not obtained because this article contains no personal information that could identify the patients. The protocol was approved by the institutional ethics and research committee, registration number GAS-1014-13/15-1.
ResultsA total of 90 patients and biopsies were evaluated. We excluded 10 patients because their paraffin blocks were missing, 16 patients because their medical records were incomplete, and 4 patients because they presented with a concomitant liver disease. Thus, 60 patients and biopsies were included in the study. The demographic, biochemical, and serologic characteristics of the patients are shown in Table 1. Median age was 39.5 (IQR, 29-48) years, with a predominance of females (75%). Five patients had type 2 AIH, according to the presence of ALKM-1. The median follow-up time was 108 (IQR, 72-156) months. Five patients had cirrhosis at diagnosis, 17 patients developed cirrhosis during follow-up, and 2 patients died due to liver related etiologies (one for variceal bleeding and one for cellulitis and septic shock).
Demographic, clinical, and biochemical characteristics of patients with autoimmune hepatitis.
| Parameters, median (IQR) | AIH (n = 60) |
|---|---|
| Age, years | 39.5 (29-48) |
| Female, n (%) | 45 (75) |
| Total bilirubin (mg/dl) | 1.45 (0.87-6.52) |
| Direct bilirubin (mg/dl) | 0.43 (0.19-3.07) |
| ALT (U/l) | 167.5 (55.25-515) |
| AST (U/l) | 179.5 (62.75-457.75) |
| ALP (U/l) | 153 (110-216.5) |
| Albumin (g/dl) | 3.33 (2.66-3.8) |
| Immunoglobulin G (g/l) | 2200 (1566.25-3307.5) |
| Leukocytes (m/mm3) | 6.15 (4.62-7.67) |
| Hemoglobin (g/dl) | 13.6 (12.52-15.27) |
| Platelets (m/mm3) | 197 (154.5-272.75) |
| Creatinine (mg/dl) | 0.68 (0.59-0.84) |
| INR | 1.1 (1-1.23) |
| ANA > 1:80, n/total (%) | 58/60 (96.6) |
| ASMA > 1:80, n/total (%) | 4/60 (6.6) |
| ALKM-1 > 1:40, n/total (%) | 5/60 (8.3) |
AIH: autoimmune hepatitis; ALKM-1: anti-liver-kidney microsomal type 1 antibodies; ALP: alkaline phosphatase; ALT: alanine aminotransferase; ANA: antinuclear antibodies; ASMA: anti-smooth muscle antibodies; AST: aspartate aminotransferase; INR: international normalized ratio; IQR: interquartile range.
All liver biopsies showed interface hepatitis and cellular infiltrates in the portal tracts. All patients with AIH had plasma cells determined through H&E staining and CD138 IHC. The number of plasma cells was higher in the CD138 group (10 [6–20] cells/high power field [HPF] versus 6 [4-9] cells/HPF, p < 0.001). There was a significant correlation between the number of plasma cells determined through H&E staining and CD138 IHC (p = 0.31, p = 0.01).
No significant correlation was found between the number of plasma cells determined by CD138 staining and IgG level (p = 0.21, p = 0.09) or stage of fibrosis (p = 0.12, p = 0.35), or between IgG level and stage of fibrosis (p = 0.17, p = 0.17) (Fig. 1).
Treatment response was complete in 29 patients and incomplete in 7. Twenty-two patients had treatment failure, and 2 patients were lost to follow-up. No significant correlation was found between treatment response and the number of plasma cells determined by H&E staining (p = 0.11, p = 0.38), CD138 IHC (p = 0.07, p = 0.55), or stage of fibrosis (p = 0.16, p = 0.20). CD138 IHC expression was different between the treatment response groups (p = 0.04): in patients with remission, the median was 10 (IQR 6-20); in cases of incomplete response to treatment, it was 6 (IQR 3-7); and in patients with treatment failure, it was 15 (IQR 8-20).
DiscussionIn the present study, we demonstrated that CD138 IHC increased the detection of plasma cells, when compared with H&E staining. However, no significant association was found between the number of plasma cells determined by CD138 IHC and serum IgG levels, the stage of fibrosis, or the response to treatment.11 To the best of our knowledge, ours is the first study to compare H&E staining versus CD138 IHC in AIH. We observed plasma cell infiltration in all cases of Mexican patients with AIH, determined through both H&E staining and CD138 IHC.
Plasma cells are prominent in 66% of patients with AIH and are a key histologic finding, but they are also common in other liver diseases. Patients with nonalcoholic steatohepatitis show increased portal inflammation with plasma cells, interface hepatitis, cholangiocyte injury, and prominent ductular reaction.12 AIH and primary biliary cholangitis (PBC) can mimic one another quite easily; both show portal inflammation with plasma cells and interface hepatitis, but AIH shows lobular inflammation and does not cause florid duct damage. Overlap syndromes, such as AIH with features of PBC, show interface hepatitis, lymphocytic portal infiltrate, portal plasma cells, and destructive cholangitis, as well.
Brandao et al. found a positive significant correlation between stellate cells, fibrosis, and the number of plasma cells. They also found a co-localization of plasma cells and activated stellate cells, suggesting that plasma cells could be a surrogate marker of disease severity, intimately related to the number of hepatic stellate cells and the stage of fibrosis.6 However, in our study, those results were not reproduced. There was no association between the number of plasma cells identified by CD138 IHC and the stage of fibrosis, which could be due to the fact that fibrosis probably depends more on the time of evolution rather than the intensity of plasma cell infiltration. Interestingly, we found a higher number of plasma cells in patients with treatment failure, which could have important prognostic implications, when starting treatment. Regos et al. reported that CD138 expression is significantly enhanced in cirrhotic liver samples. Furthermore, we found greater expression in the group of patients with treatment failure that already had cirrhosis or that developed it.13
Some of the limitations of the present study are its retrospective design, its small sample size, and the fact that liver biopsy examination is subject to sampling error and inter and intra-observer variability. Nevertheless, large inconsistencies in standardized semi-quantitative scores have been reported to be low, if the assessment is performed by an expert pathologist, as was the case in our study.
In conclusion, CD138 IHC increased the detection of plasma cells in liver biopsies of patients with AIH, when compared with routine H&E staining. However, there was no correlation between the number of plasma cells determined by CD138 IHC and serum IgG levels, stage of fibrosis, or response to treatment.
Ethical considerationsThe authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Financial disclosureNo specific grants were received from public sector agencies, the business sector, or non-profit organizations in relation to this study.
Conflict of interestThe authors declare that there is no conflict of interest.