Gastric cancer is one of the main causes of cancer worldwide, but there is currently no global screening strategy for the disease. Endoscopy is the screening method of choice in some Asian countries, but no standardized technique has been recognized. Systematic alphanumeric-coded endoscopy can increase gastric lesion detection. The aim of the present article was to compare the usefulness of systematic alphanumeric-coded endoscopy with conventional endoscopy for the detection of premalignant lesions and early gastric cancer in subjects at average risk for gastric cancer.
Materials and methodsA cross-sectional, comparative, prospective, randomized study was conducted on patients at average risk for gastric cancer (40-50 years of age, no history of H. pylori infection, intestinal metaplasia, gastric atrophy, or gastrointestinal surgery). Before undergoing endoscopy, the patients had gastric preparation (200mg of oral acetylcysteine or 50mg of oral dimethicone). Conventional chromoendoscopy was performed with indigo carmine dye for contrast enhancement.
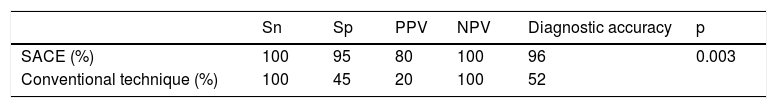
ResultsFifty consecutive cases (mean age 44.4 ± 3.34 years, 60% women, BMI 27.6 ± 5.82kg/m2) were evaluated. Endoscopic imaging quality was satisfactory in all the cases, with no differences between methods (p = 0.817). The detection rate of premalignant lesions and early gastric cancer was 14% (6 cases of intestinal metaplasia and one case of gastric adenocarcinoma). Sensitivity, specificity, positive predictive value, negative predictive value, and diagnostic accuracy were 100, 95, 80, 100 and 96%, respectively, for systematic alphanumeric-coded endoscopy, and 100, 45, 20, 100, and 52%, respectively, for conventional endoscopy. Lesion detection through systematic alphanumeric-coded endoscopy was superior to that of conventional endoscopy (p = 0.003; OR = 12).
ConclusionBoth techniques were effective, but systematic alphanumeric-coded endoscopy significantly reduced the false positive rate.
El cáncer gástrico es una de las principales causas de cáncer a nivel mundial. No existe una estrategia global de tamizaje. La endoscopia se ha convertido en el método de elección para tamizaje, sin embargo, no existe un método estandarizado. El uso de la endoscopia sistemática alfanumérica codificada puede aumentar la detección de lesiones gástricas. El objetivo es comparar la utilidad del sistema de endoscopia alfanumérica codificada con la endoscopia convencional para la detección de lesiones premalignas y cáncer gástrico temprano en sujetos con riesgo promedio de cáncer gástrico.
Material y métodosEstudio de corte transversal, comparativo, prospectivo y aleatorizado, en pacientes con riesgo promedio para cáncer gástrico (40-50 años, sin historia de infección por H. pylori, metaplasia intestinal, atrofia gástrica ni cirugía gastrointestinal). Antes de la endoscopia recibieron preparación gástrica (200mg de acetilcisteína oral o 50mg de dimeticona oral). Se realizó cromoendoscopia convencional con índigo carmín para realzar el contraste.
ResultadosFueron 50 casos consecutivos (edad promedio de 44.4 ± 3.34 años, el 60% mujeres 60%, con IMC de 27.6 ± 5.82kg/cm2). La calidad de la imagen endoscópica fue satisfactoria en todos los casos, sin diferencias entre métodos (p = 0.817). El índice de detección de lesiones premalignas y de cáncer gástrico temprano fue del 14% (6 metaplasias intestinales y un adenocarcinoma gástrico). La Sn, Sp, VPP, VPN y la exactitud diagnóstica fueron del 100, 95, 80, 100 y 96%, respectivamente para la endoscopia sistemática alfanumérica codificada y para la endoscopia convencional 100, 45, 20, 100 y 52%, respectivamente. La endoscopia sistemática alfanumérica codificada fue mejor que la endoscopia convencional para la detección de lesiones (p = 0.003; RM = 12).
ConclusiónAmbas técnicas fueron efectivas; sin embargo, el sistema alfanumérico codificado disminuye significativamente la tasa de falsos positivos.
Gastric cancer is the third cause of cancer death (723,000 deaths/year), with 870,000 new cases reported in 2001.1,2 There is currently no universally accepted screening strategy for premalignant lesions.3 Both noninvasive techniques (radiologic methods, H. pylori serology, and pepsinogen I, pepsinogen II, and serum gastrin determinations) and invasive techniques (endoscopy, magnification, chromoendoscopy, narrow band imaging, autofluorescence, and confocal laser endomicroscopy) have been proposed.3
Intestinal metaplasia and gastric atrophy and dysplasia increase the risk for cancer.4 The Paris Classification system has been accepted worldwide as a unified way to classify gastric lesions.5 The interval gastric cancer rate (23.1%) has been related to lesion size and the presence of intestinal metaplasia.6–8 Thorough examination and adequate cleansing of the gastric mucosa are essential for detecting said lesions in at-risk subjects.9
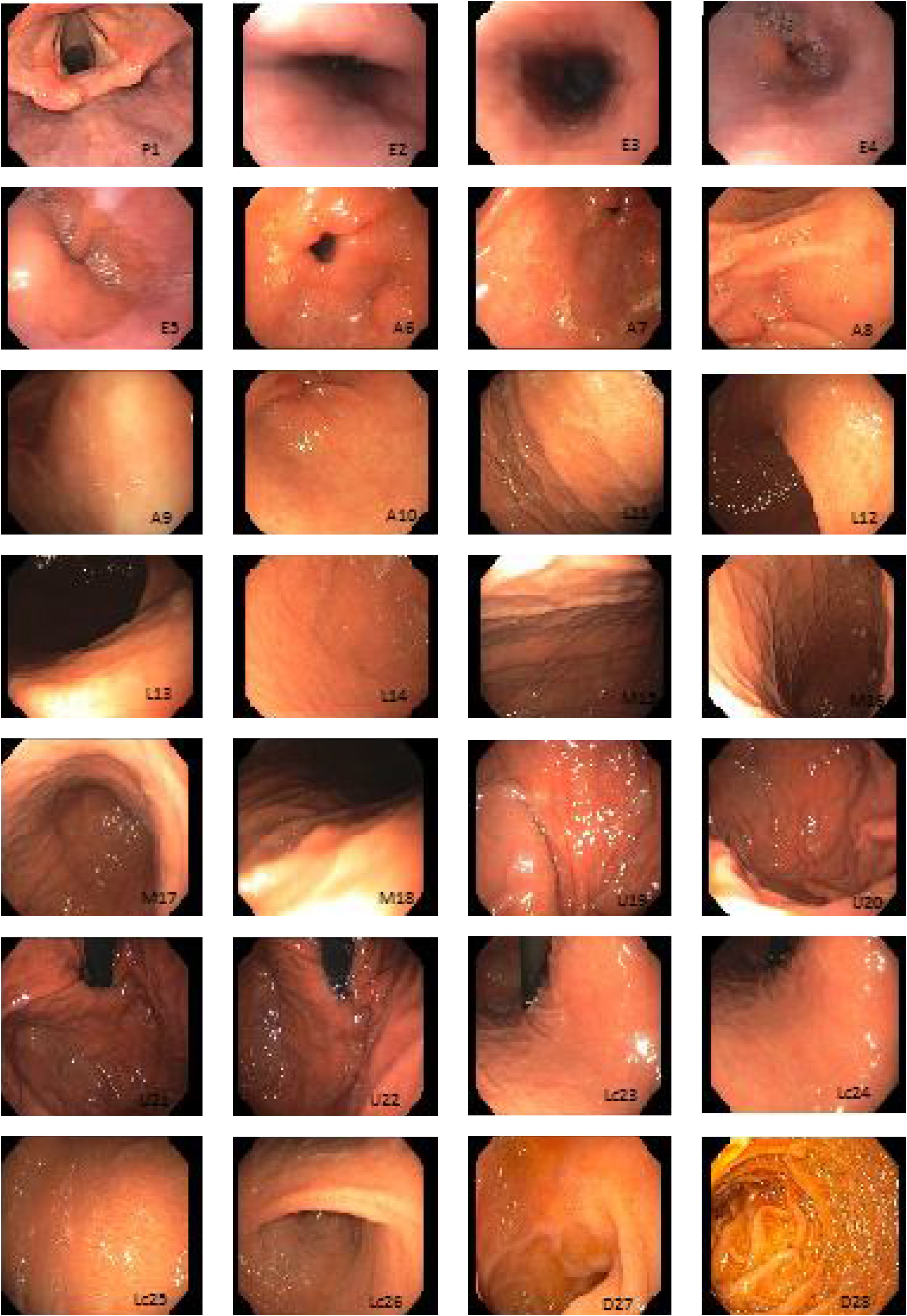
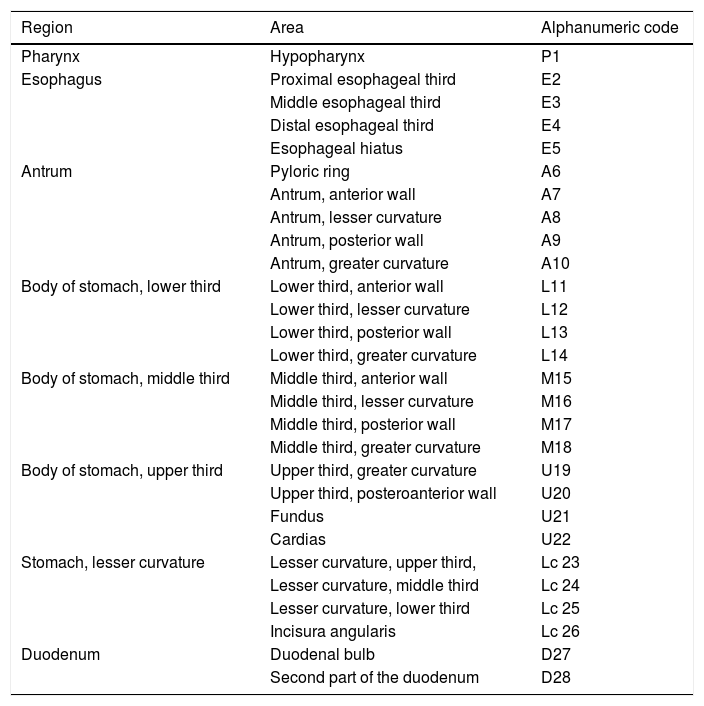
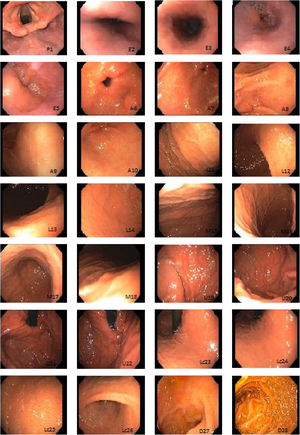
Systematic alphanumeric-coded endoscopy (SACE) is a method for evaluating the gastric mucosa that divides the upper digestive tract into 8 regions and 28 areas (Table 1, fig. 1). It enables the examination of the entire surface through a system of coordinates based on the identification of certain natural axes, walls, curvatures, and anatomic reference points, preventing blind spots, and thus having a significant impact on the detection rate of premalignant lesions and the reduction of interval cancer.9–11
Systematic alphanumeric-coded endoscopy.
| Region | Area | Alphanumeric code |
|---|---|---|
| Pharynx | Hypopharynx | P1 |
| Esophagus | Proximal esophageal third | E2 |
| Middle esophageal third | E3 | |
| Distal esophageal third | E4 | |
| Esophageal hiatus | E5 | |
| Antrum | Pyloric ring | A6 |
| Antrum, anterior wall | A7 | |
| Antrum, lesser curvature | A8 | |
| Antrum, posterior wall | A9 | |
| Antrum, greater curvature | A10 | |
| Body of stomach, lower third | Lower third, anterior wall | L11 |
| Lower third, lesser curvature | L12 | |
| Lower third, posterior wall | L13 | |
| Lower third, greater curvature | L14 | |
| Body of stomach, middle third | Middle third, anterior wall | M15 |
| Middle third, lesser curvature | M16 | |
| Middle third, posterior wall | M17 | |
| Middle third, greater curvature | M18 | |
| Body of stomach, upper third | Upper third, greater curvature | U19 |
| Upper third, posteroanterior wall | U20 | |
| Fundus | U21 | |
| Cardias | U22 | |
| Stomach, lesser curvature | Lesser curvature, upper third, | Lc 23 |
| Lesser curvature, middle third | Lc 24 | |
| Lesser curvature, lower third | Lc 25 | |
| Incisura angularis | Lc 26 | |
| Duodenum | Duodenal bulb | D27 |
| Second part of the duodenum | D28 |
The aim of the present study was to compare the usefulness of SACE with conventional endoscopy in the detection of premalignant lesions and early gastric cancer in subjects at average risk for gastric cancer. We defined patients at average risk as those between the ages of 40 and 50 years, with no associated factor that increased the risk for cancer.
Materials and methodsA cross-sectional, randomized, prospective study was conducted on patients at average risk for gastric cancer at the Hospital General de México “Dr. Eduardo Liceaga”.
Selection criteria: a) inclusion criteria: healthy subjects between 40 and 50 years of age, both sexes, signed statements of informed consent, b) exclusion criteria: history of H. pylori infection, intestinal metaplasia, gastric atrophy or dysplasia, gastric surgery with resection, bowel obstruction, portal hypertension, and gastrointestinal bleeding.
PremedicationAll patients underwent pre-endoscopic gastric cleansing (randomly assigned) through oral intake (20-30min before the procedure) of dimethicone suspension (Espaven Pediatrics®, 50mg) or N-acetylcysteine (200mg of granulated Lysomucil®) diluted in 100ml of water. Gastric cleansing quality was evaluated through the following scores: 0 = no residue, the mucosa can be completely evaluated; 1 = some residue, all of which can be aspirated with the endoscope; 2 = thick liquid residue, needing irrigation with water to be dissolved, followed by complete aspiration; 3 = thick liquid residue, needing irrigation with water to be dissolved, followed by incomplete aspiration; 4 = solid residue that cannot be aspirated. The mean endoscopic study duration required was registered.
The patients were randomly assigned (random number table) to one of the two gastric examination groups: 1) conventional endoscopy or 2) SACE.
Endoscopy equipmentGIF-Q145 (Japan 2000) and GIF-Q150 (Japan 2006) video gastroscopes, EVIS EXERA-II processor.
Chromoendoscopy and biopsyThe studies were performed by physicians in endoscopy training and supervised by staff physicians. In the SACE group, images were taken upon entrance, after completing additional cleansing of the gastric chamber (if necessary) to expose 100% of the mucosa, following the previously described coding (Table 1, fig. 1). Premalignant lesion was defined as a lesion having any of the following characteristics: polypoid or ulcerous, Paris 0-IIa and 0-IIc lesions, change in coloring (reddish or whitish), or markedly altered flat, pale mucosal areas. If any lesion was suspected of being premalignant, 0.3% indigo carmine was sprayed on it and biopsies were taken. They were immediately fixed in 10% formaldehyde and evaluated by hospital pathologists. If no premalignant lesions were suspected, but there were signs of chronic gastropathy (diffuse antral gastritis, diffuse corporal atrophic gastritis, or multifocal atrophic gastritis), systematic biopsies were taken, according to the Sydney protocol.12
Statistical analysisThe lesion detection rate was calculatedDescriptive statistics for frequencies and proportions were carried out. The chi-squared test was used for the categorical variables and the Student's t test for the quantitative variables, with a significance level of 0.05. The size of the effect (odds ratio with 95% CI) was also calculated. Sample size was established at 23 subjects per group through the proportions formula (α = 0.05, β =0.2, power 0.8, δ = 0.4).
The study protocol was reviewed and accepted by the Research and Ethics Committee of the hospital (DI/16/107/03/032) and the Good Clinical Practice Guidelines were followed.
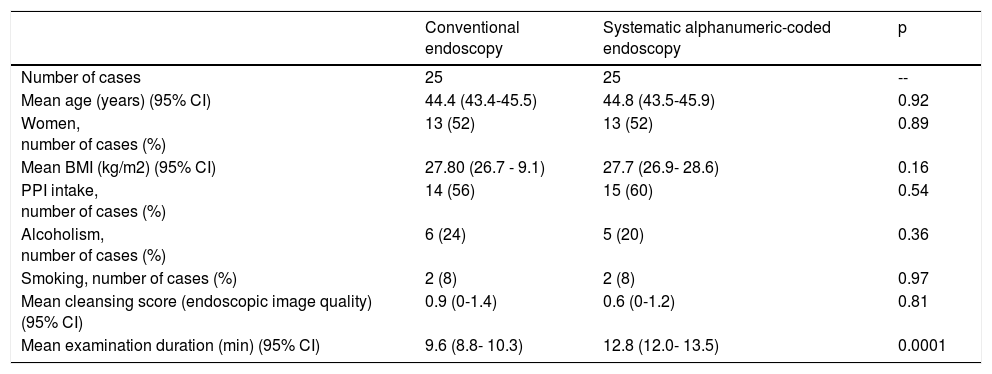
ResultsThere were 50 consecutive cases, mean patient age was 44.4 ± 3.34 years, 52% of the patients were women, and mean BMI was 27.6 ± 5.82kg/cm2. Background histories: 58% of the patients took PPIs, 22% were alcoholics, and 8% were smokers. There were no differences in relation to age (p = 0.92) or BMI (p = 0.16) between groups. Endoscopic image quality for the morphologic differentiation of premalignant lesions was satisfactory in all the cases. No differences in the endoscopic image quality were observed between groups (p = 0.817).
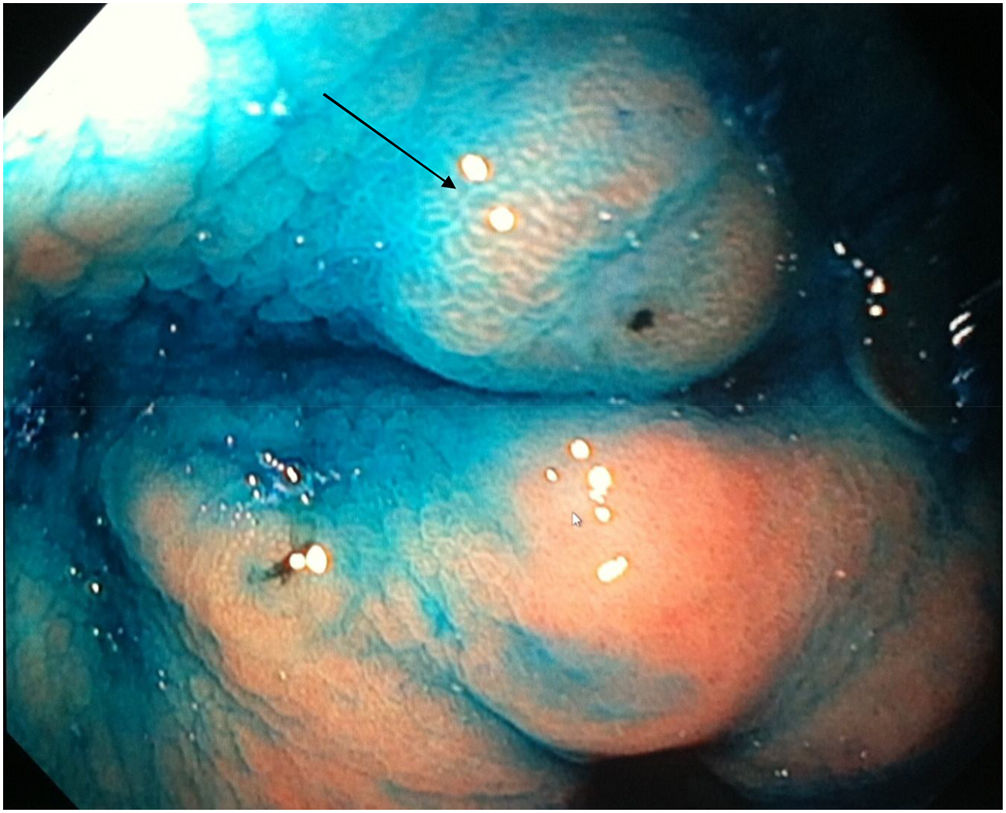
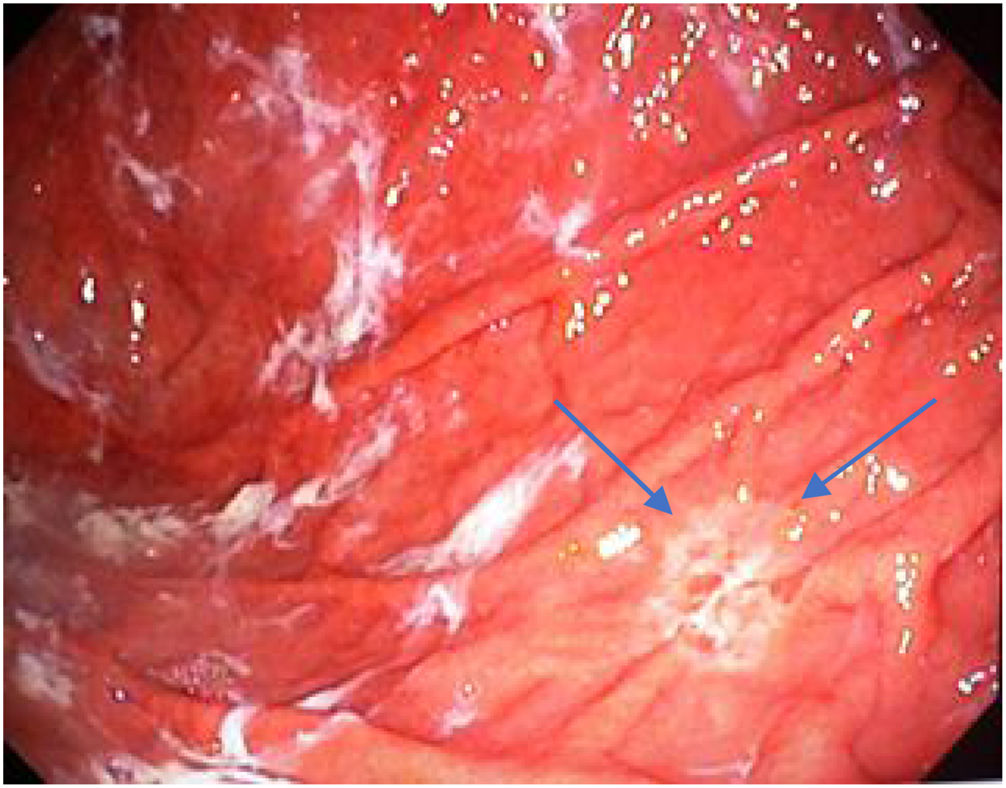
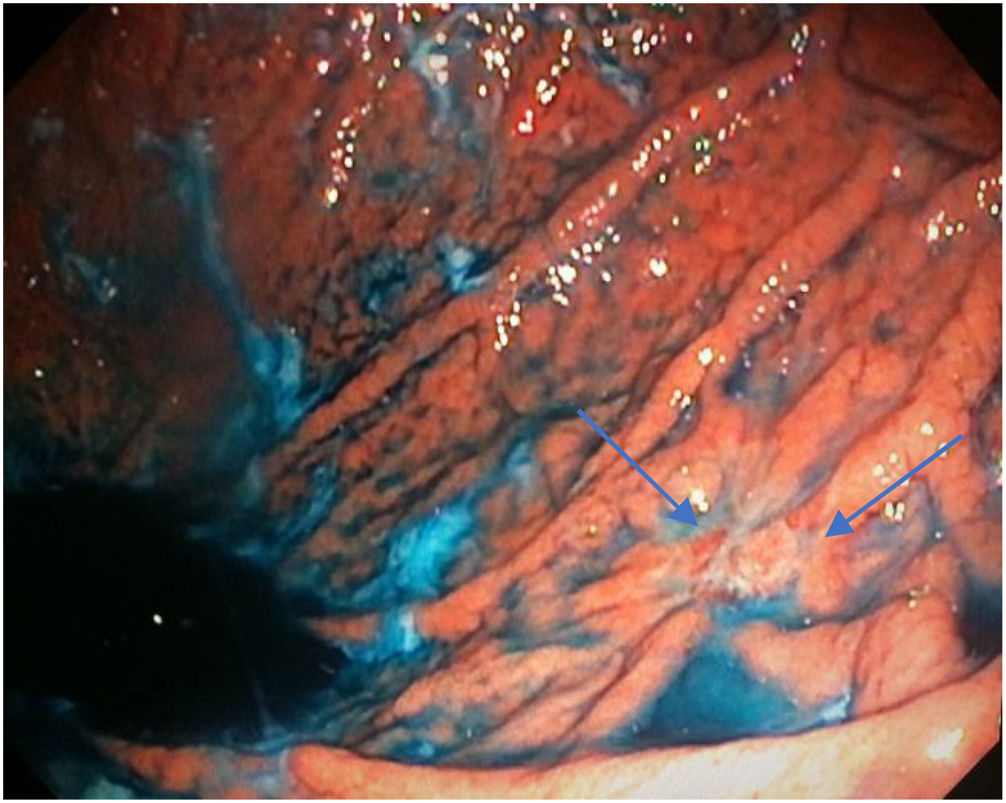
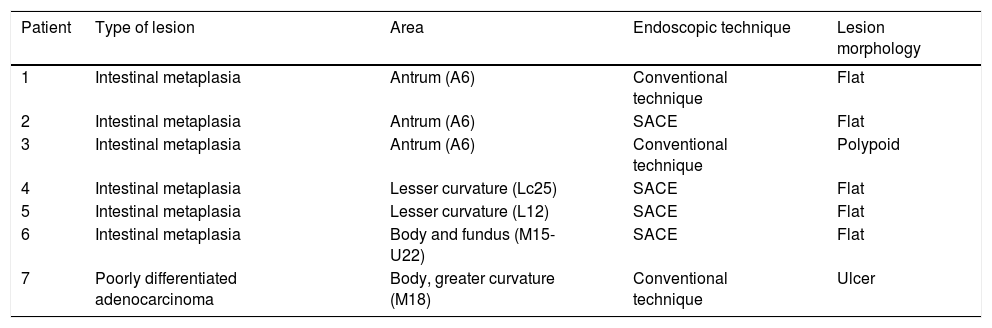
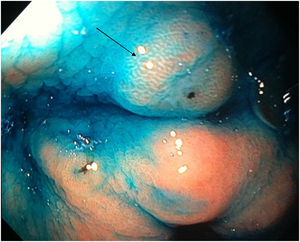
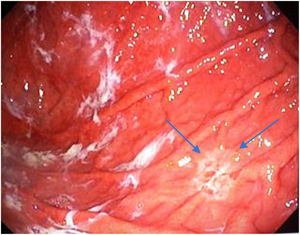
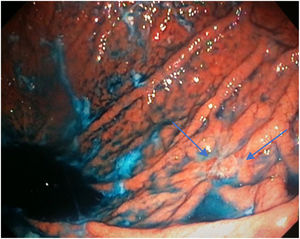
Mean duration (minutes) of the mucosal examination was significantly longer with SACE (12.8 ± 2.6, 95% CI: 12.09-13.51 versus 9.6 ± 2.42, 95% CI: 8.88-10.31; p = 0.0001) (Table 2). Endoscopic findings (histologic confirmation): 18% (9/50) of the patients had normal mucosa, 20% (10/50) had chronic gastritis associated with H. pylori, 6% (3/50) had reactive gastritis, and 56% (28/50) had chronic gastritis. Six cases of intestinal metaplasia with no dysplasia (figs. 2 and 3) and one case of poorly differentiated adenocarcinoma (diffuse, according to the Lauren classification) that presented as a flat, ulcerated lesion (fig. 4, Table 3) were detected. The detection rate for premalignant lesions and gastric cancer was 14%. The most frequent region and areas with lesions were the antrum and lesser curvature. Sensitivity (Sn), specificity (Sp), positive predictive value (PPV), negative predictive value (NPV), and diagnostic accuracy were 100, 95, 80, 100, and 96%, respectively, for SACE. Sn, Sp, PPV, NPV, and diagnostic accuracy were 100, 45, 20, 100, and 52%, respectively, for conventional endoscopy. SACE was superior to conventional endoscopy for detecting pre-neoplastic lesions (p = 0.03; OR = 12) (Table 4). Interobserver agreement was low (kappa 0.4).
Demographic characteristics of the study population.
| Conventional endoscopy | Systematic alphanumeric-coded endoscopy | p | |
|---|---|---|---|
| Number of cases | 25 | 25 | -- |
| Mean age (years) (95% CI) | 44.4 (43.4-45.5) | 44.8 (43.5-45.9) | 0.92 |
| Women, number of cases (%) | 13 (52) | 13 (52) | 0.89 |
| Mean BMI (kg/m2) (95% CI) | 27.80 (26.7 - 9.1) | 27.7 (26.9- 28.6) | 0.16 |
| PPI intake, number of cases (%) | 14 (56) | 15 (60) | 0.54 |
| Alcoholism, number of cases (%) | 6 (24) | 5 (20) | 0.36 |
| Smoking, number of cases (%) | 2 (8) | 2 (8) | 0.97 |
| Mean cleansing score (endoscopic image quality) (95% CI) | 0.9 (0-1.4) | 0.6 (0-1.2) | 0.81 |
| Mean examination duration (min) (95% CI) | 9.6 (8.8- 10.3) | 12.8 (12.0- 13.5) | 0.0001 |
BMI: Body mass index; CI: Confidence interval; PPI: Proton pump inhibitor.
Cases with pre-neoplastic lesions.
| Patient | Type of lesion | Area | Endoscopic technique | Lesion morphology |
|---|---|---|---|---|
| 1 | Intestinal metaplasia | Antrum (A6) | Conventional technique | Flat |
| 2 | Intestinal metaplasia | Antrum (A6) | SACE | Flat |
| 3 | Intestinal metaplasia | Antrum (A6) | Conventional technique | Polypoid |
| 4 | Intestinal metaplasia | Lesser curvature (Lc25) | SACE | Flat |
| 5 | Intestinal metaplasia | Lesser curvature (L12) | SACE | Flat |
| 6 | Intestinal metaplasia | Body and fundus (M15-U22) | SACE | Flat |
| 7 | Poorly differentiated adenocarcinoma | Body, greater curvature (M18) | Conventional technique | Ulcer |
Gastric cancer is one of the main causes of cancer deaths, worldwide. Most cases are diagnosed at advanced stages and have a poor prognosis. Precancerous lesions include intestinal metaplasia and gastric atrophy. The authors of a Danish study showed an increased risk for cancer in patients with premalignant gastric lesions, reporting an annual incidence of gastric cancer of: 0.1% for patients with gastric atrophy, 0.25% for intestinal metaplasia, 0.6% for moderate dysplasia, and 0.6% for severe dysplasia, within 5 years from diagnosis.4 Despite that prevalence, a global screening strategy does not exist.3 In some high-prevalence zones, suggestions have been made to begin screening in men and women above 40 years of age, but that age range must be adjusted in specific populations, depending on gastric cancer incidence and individual risk factors.13,14 In non-endemic areas, it is recommended to begin screening at 40-45 years of age.15
Endoscopy has become the method of choice in Japan and Korea, with reasonable cost-benefit. However, cost continues to be a problem in developing countries, in addition to the inherent risks of an invasive study. New imaging techniques, such as digital chromoendoscopy, magnification endoscopy, narrow band imaging, autofluorescence, confocal laser endomicroscopy, or endocystoscopy can improve premalignant lesion detection.16–20 However, those techniques are not available at most endoscopy units. The detection of premalignant lesions depends on the knowledge and skill of the endoscopist, which is why the standardization of a technique and adequate training are important.21 Hosokawa et al. reported false negative rates of up to 25.5% in early gastric cancer detection with endoscopy, and Aida et al. showed that gastric cancer lesions were undetected in a previous endoscopy in 23.1% of the cases.7,22 In a study that evaluated the factors related to the development of interval gastric cancer (cancer detected within 2 years of a negative endoscopy), the smallest lesions (1.3cm vs 1.8cm) and the presence of intestinal metaplasia were statistically significant in the multivariate analysis,8 signifying that adequate endoscopic examination is necessary to detect those types of lesions.
The SACE protocol proposed by Emura et al. is a method for systematically inspecting the entire surface of the upper gastrointestinal tract based on the sequential photo-documentation of partially overlapped images, using an endoluminal alphanumeric-coded nomenclature for 8 regions and 28 areas of the digestive tract. It incorporates a simple system of coordinates based on the identification of certain natural axes, walls, curvatures, and anatomic reference points (Table 1, fig. 1). The effectiveness of the technique was demonstrated in a screening study on healthy, average-risk volunteers between 40 and 70 years of age, in which 0.3% (2/650) of the patients were diagnosed with early gastric cancer.22 The SACE system has not been evaluated in the Mexican population. Ideally, a screening test should include intraluminal premedication, high-definition equipment, digital chromoendoscopy, and endoscopists with visual experience in image identification.23 In the present case series, premedication was carried out with dimethicone or acetylcysteine to remove the adhered mucus and foam produced by secretions, which favored adequate evaluation of the gastric mucosal surface. The 2 preparations were analyzed (data not shown) and the dimethicone group had better endoscopic image quality. Conventional chromoendoscopy with indigo carmine dye (at 0.3%), which is non-absorbable and non-toxic, has shown superior detection of pre-neoplastic lesions and a facility for characterizing the lesions, their edges, and size.23 In our study, there was a 14% prevalence of premalignant lesions and early gastric cancer, which is lower than that reported in populations considered to have high prevalence, such as Colombia (30%).9 It was similar to prevalence reported in countries like Turkey (13%).24 Mexico is regarded as a population with a low prevalence of intestinal metaplasia and gastric cancer. Both endoscopic techniques were efficacious for detecting cases (100%), but there was a high false positive rate (95% Sn vs 45% Sn) and a very low PPV (80 vs 20%) with the conventional method. The SACE technique was more effective than the conventional method in detecting premalignant lesions and early gastric cancer (p = 0.003; OR = 12; 95% CI). The limitations of our study were the low prevalence of those types of lesions in the study population, not having used high-definition endoscopes to characterize the lesions, and low interobserver agreement. In addition, it was not a cross-over study with the patient as his or her own control to determine the rate of undetected lesions with the two techniques. The strengths of our study were based on its methodology (cross-sectional design with randomized assignation). To the best of our knowledge, this is the only screening study evaluating the performance of SACE in Mexico. Ideally, endoscopic centers should utilize high-definition equipment and electronic chromoendoscopy to screen average-risk and high-risk subjects for gastric cancer. Nevertheless, systematic evaluation is a tool that can have a significant impact on lesion omission and interval cancer reduction.
In conclusion, SACE and conventional endoscopy were both effective in detecting premalignant lesions, but the alphanumeric-coded system significantly reduced the false positive rate.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the responsible Clinical Research Ethics Committee and in accordance with those of the World Medical Association and the Helsinki Declaration.
Confidentiality of dataThe authors declare that they have adhered to the protocols of their centre of work on patient data publication.
Right to privacy and informed consentThe authors must have obtained the informed consent of the patients and/or subjects mentioned in the article. The author for correspondence must be in possession of this document.
Financial disclosureNo financial support was received in relation to this study/article.
Conflict of interestThe authors declare that there is no conflict of interest.
The authors wish to thank the staff physicians, residents, and nurses whose participation made this study possible.
Please cite this article as: Pérez-Mendoza A, Zárate-Guzmán ÁM, Galvis García ES, Sobrino Cossío S, Djamus Birch J. Aplicación de la endoscopia sistemática alfanumérica codificada más cromoendoscopia para la detección de lesiones precancerosas gástricas y cáncer gástrico temprano en sujetos con riesgo promedio de cáncer gástrico. Revista de Gastroenterología de México. 2018;83:117–124.